Double-jabbed’ Malhotra originally supported the program, until a series of events sent him digging into the evidence. What he discovered alarmed him and resulted in the publication of two evidence-based, peer-reviewed papers along with a call for the immediate suspension of the Covid mRNA roll-out…
Over following months, emerging data led Malhotra to question whether the vaccine was linked to his father’s death. The first was an abstract published in Circulation (November 8, 2021) by US cardiothoracic surgeon, Dr Steven Gundry, who followed several hundred of his patients after the mRNA (Moderna/Pfizer) jabs. Gundry found that inflammatory markers correlated with heart disease risk went through the roof. On average, that change increased the risk of those people having a heart attack or stroke within the next five years, from 11 per cent up to 25 per cent. This increase in risk is massive.
Safety
Browse the articles related to this topic below.
Join our community on Guilded.
Something strange is going on with the VAERS system. Reports that were present three months ago are now inexplicably missing. And fewer than 4% of adverse events recorded in V-Safe have made their way to VAERS. This is the CDC’s database; Dr. Rochelle Walensky is in charge of it. And the agency’s failure to properly manage VAERS is suppressing the already-alarming safety signal of the Covid-19 shots.
Those who have received the vaccine should, however, examine their conscience and ask themselves what exactly they knew at the time. If they knew nothing about the ethical issues, was this for lack of having taking the trouble to find out? Ignorance is sometimes culpable. However in those who have a duty to know (priests, doctors, government officials, judges), it is always culpable.
In an exclusive and explosive one-hour interview with Veronika Kyrylenko of The New American, pioneering mRNA scientist Dr. Robert Malone explains the intensely corrupt workings of the government regulatory bodies that have mismanaged the pandemic, discusses the problems with the vaccine program and delves into potentially explosive and game-changing revelations about the shady origins of the Covid-19 pandemic in Wuhan, China.
Revelations of poor practices at a contract research company helping to carry out Pfizer’s pivotal covid-19 vaccine trial raise questions about data integrity and regulatory oversight. Paul D Thacker reports
In autumn 2020 Pfizer’s chairman and chief executive, Albert Bourla, released an open letter to the billions of people around the world who were investing their hopes in a safe and effective covid-19 vaccine to end the pandemic. “As I’ve said before, we are operating at the speed of science,” Bourla wrote, explaining to the public when they could expect a Pfizer vaccine to be authorised in the United States.1
But, for researchers who were testing Pfizer’s vaccine at several sites in Texas during that autumn, speed may have come at the cost of data integrity and patient safety. A regional director who was employed at the research organisation Ventavia Research Group has told The BMJ that the company falsified data, unblinded patients, employed inadequately trained vaccinators, and was slow to follow up on adverse events reported in Pfizer’s pivotal phase III trial. Staff who conducted quality control checks were overwhelmed by the volume of problems they were finding. After repeatedly notifying Ventavia of these problems, the regional director, Brook Jackson, emailed a complaint to the US Food and Drug Administration (FDA). Ventavia fired her later the same day. Jackson has provided The BMJ with dozens of internal company documents, photos, audio recordings, and emails.
Poor laboratory management
On its website Ventavia calls itself the largest privately owned clinical research company in Texas and lists many awards it has won for its contract work.2 But Jackson has told The BMJ that, during the two weeks she was employed at Ventavia in September 2020, she repeatedly informed her superiors of poor laboratory management, patient safety concerns, and data integrity issues. Jackson was a trained clinical trial auditor who previously held a director of operations position and came to Ventavia with more than 15 years’ experience in clinical research coordination and management. Exasperated that Ventavia was not dealing with the problems, Jackson documented several matters late one night, taking photos on her mobile phone. One photo, provided to The BMJ, showed needles discarded in a plastic biohazard bag instead of a sharps container box. Another showed vaccine packaging materials with trial participants’ identification numbers written on them left out in the open, potentially unblinding participants. Ventavia executives later questioned Jackson for taking the photos.
Early and inadvertent unblinding may have occurred on a far wider scale. According to the trial’s design, unblinded staff were responsible for preparing and administering the study drug (Pfizer’s vaccine or a placebo). This was to be done to preserve the blinding of trial participants and all other site staff, including the principal investigator. However, at Ventavia, Jackson told The BMJ that drug assignment confirmation printouts were being left in participants’ charts, accessible to blinded personnel. As a corrective action taken in September, two months into trial recruitment and with around 1000 participants already enrolled, quality assurance checklists were updated with instructions for staff to remove drug assignments from charts.
In a recording of a meeting in late September2020 between Jackson and two directors a Ventavia executive can be heard explaining that the company wasn’t able to quantify the types and number of errors they were finding when examining the trial paperwork for quality control. “In my mind, it’s something new every day,” a Ventavia executive says. “We know that it’s significant.”
Ventavia was not keeping up with data entry queries, shows an email sent by ICON, the contract research organisation with which Pfizer partnered on the trial. ICON reminded Ventavia in a September 2021 email: “The expectation for this study is that all queries are addressed within 24hrs.” ICON then highlighted over 100 outstanding queries older than three days in yellow. Examples included two individuals for which “Subject has reported with Severe symptoms/reactions … Per protocol, subjects experiencing Grade 3 local reactions should be contacted. Please confirm if an UNPLANNED CONTACT was made and update the corresponding form as appropriate.” According to the trial protocol a telephone contact should have occurred “to ascertain further details and determine whether a site visit is clinically indicated.”
Worries over FDA inspection
Documents show that problems had been going on for weeks. In a list of “action items” circulated among Ventavia leaders in early August 2020, shortly after the trial began and before Jackson’s hiring, a Ventavia executive identified three site staff members with whom to “Go over e-diary issue/falsifying data, etc.” One of them was “verbally counseled for changing data and not noting late entry,” a note indicates. At several points during the late September meeting Jackson and the Ventavia executives discussed the possibility of the FDA showing up for an inspection (box 1). “We’re going to get some kind of letter of information at least, when the FDA gets here . . . know it,” an executive stated.
Box 1
A history of lax oversight
When it comes to the FDA and clinical trials, Elizabeth Woeckner, president of Citizens for Responsible Care and Research Incorporated (CIRCARE),3 says the agency’s oversight capacity is severely under-resourced. If the FDA receives a complaint about a clinical trial, she says the agency rarely has the staff available to show up and inspect. And sometimes oversight occurs too late.
In one example CIRCARE and the US consumer advocacy organisation Public Citizen, along with dozens of public health experts, filed a detailed complaint in July 2018 with the FDA about a clinical trial that failed to comply with regulations for the protection of human participants.4 Nine months later, in April 2019, an FDA investigator inspected the clinical site. In May this year the FDA sent the triallist a warning letter that substantiated many of the claims in the complaints. It said, “[I]t appears that you did not adhere to the applicable statutory requirements and FDA regulations governing the conduct of clinical investigations and the protection of human subjects.”5
“There’s just a complete lack of oversight of contract research organisations and independent clinical research facilities,” says Jill Fisher, professor of social medicine at the University of North Carolina School of Medicine and author of Medical Research for Hire: The Political Economy of Pharmaceutical Clinical Trials.
Ventavia and the FDA
A former Ventavia employee told The BMJ that the company was nervous and expecting a federal audit of its Pfizer vaccine trial.
“People working in clinical research are terrified of FDA audits,” Jill Fisher told The BMJ, but added that the agency rarely does anything other than inspect paperwork, usually months after a trial has ended. “I don’t know why they’re so afraid of them,” she said. But she said she was surprised that the agency failed to inspect Ventavia after an employee had filed a complaint. “You would think if there’s a specific and credible complaint that they would have to investigate that,” Fisher said.
In 2007 the Department of Health and Human Services’ Office of the Inspector General released a report on FDA’s oversight of clinical trials conducted between 2000 and 2005. The report found that the FDA inspected only 1% of clinical trial sites.6 Inspections carried out by the FDA’s vaccines and biologics branch have been decreasing in recent years, with just 50 conducted in the 2020 fiscal year.7
The next morning, 25 September 2020, Jackson called the FDA to warn about unsound practices in Pfizer’s clinical trial at Ventavia. She then reported her concerns in an email to the agency. In the afternoon Ventavia fired Jackson—deemed “not a good fit,” according to her separation letter.
Jackson told The BMJ it was the first time she had been fired in her 20 year career in research.
Concerns raised
In her 25 September email to the FDA Jackson wrote that Ventavia had enrolled more than 1000 participants at three sites. The full trial (registered under NCT04368728) enrolled around 44 000 participants across 153 sites that included numerous commercial companies and academic centres. She then listed a dozen concerns she had witnessed, including:
-Participants placed in a hallway after injection and not being monitored by clinical staff
-Lack of timely follow-up of patients who experienced adverse events
-Protocol deviations not being reported
-Vaccines not being stored at proper temperatures
-Mislabelled laboratory specimens, and
-Targeting of Ventavia staff for reporting these types of problems.
Within hours Jackson received an email from the FDA thanking her for her concerns and notifying her that the FDA could not comment on any investigation that might result. A few days later Jackson received a call from an FDA inspector to discuss her report but was told that no further information could be provided. She heard nothing further in relation to her report.
In Pfizer’s briefing document submitted to an FDA advisory committee meeting held on 10 December 2020 to discuss Pfizer’s application for emergency use authorisation of its covid-19 vaccine, the company made no mention of problems at the Ventavia site. The next day the FDA issued the authorisation of the vaccine.8
In August this year, after the full approval of Pfizer’s vaccine, the FDA published a summary of its inspections of the company’s pivotal trial. Nine of the trial’s 153 sites were inspected. Ventavia’s sites were not listed among the nine, and no inspections of sites where adults were recruited took place in the eight months after the December 2020 emergency authorisation. The FDA’s inspection officer noted: “The data integrity and verification portion of the BIMO [bioresearch monitoring] inspections were limited because the study was ongoing, and the data required for verification and comparison were not yet available to the IND [investigational new drug].”
Other employees’ accounts
In recent months Jackson has reconnected with several former Ventavia employees who all left or were fired from the company. One of them was one of the officials who had taken part in the late September meeting. In a text message sent in June the former official apologised, saying that “everything that you complained about was spot on.”
Two former Ventavia employees spoke to The BMJ anonymously for fear of reprisal and loss of job prospects in the tightly knit research community. Both confirmed broad aspects of Jackson’s complaint. One said that she had worked on over four dozen clinical trials in her career, including many large trials, but had never experienced such a “helter skelter” work environment as with Ventavia on Pfizer’s trial.
“I’ve never had to do what they were asking me to do, ever,” she told The BMJ. “It just seemed like something a little different from normal—the things that were allowed and expected.”
She added that during her time at Ventavia the company expected a federal audit but that this never came.
After Jackson left the company problems persisted at Ventavia, this employee said. In several cases Ventavia lacked enough employees to swab all trial participants who reported covid-like symptoms, to test for infection. Laboratory confirmed symptomatic covid-19 was the trial’s primary endpoint, the employee noted. (An FDA review memorandum released in August this year states that across the full trial swabs were not taken from 477 people with suspected cases of symptomatic covid-19.)
“I don’t think it was good clean data,” the employee said of the data Ventavia generated for the Pfizer trial. “It’s a crazy mess.”
A second employee also described an environment at Ventavia unlike any she had experienced in her 20 years doing research. She told The BMJ that, shortly after Ventavia fired Jackson, Pfizer was notified of problems at Ventavia with the vaccine trial and that an audit took place.
Since Jackson reported problems with Ventavia to the FDA in September 2020, Pfizer has hired Ventavia as a research subcontractor on four other vaccine clinical trials (covid-19 vaccine in children and young adults, pregnant women, and a booster dose, as well an RSV vaccine trial; NCT04816643, NCT04754594, NCT04955626, NCT05035212). The advisory committee for the Centers for Disease Control and Prevention is set to discuss the covid-19 paediatric vaccine trial on 2 November.
Footnotes
Provenance and peer review: commissioned; externally peer reviewed.
Competing interests: PDT has been doubly vaccinated with Pfizer’s vaccine.
This article is made freely available for use in accordance with BMJ’s website terms and conditions for the duration of the covid-19 pandemic or until otherwise determined by BMJ. You may use, download and print the article for any lawful, non-commercial purpose (including text and data mining) provided that all copyright notices and trade marks are retained.
https://bmj.com/coronavirus/usage
References
* Bourla A. An open letter from Pfizer chairman and CEO Albert Bourla. Pfizer. https://www.pfizer.com/news/hot-topics/an_open_letter_from_pfizer_chairman_and_ceo_albert_bourla.
* Ventavia. A leading force in clinical research trials. https://www.ventaviaresearch.com/company.
* Citizens for Responsible Care and Research Incorporated (CIRCARE). http://www.circare.org/corp.htm.
* Public Citizen. Letter to Scott Gottlieb and Jerry Menikoff. Jul 2018. https://www.citizen.org/wp-content/uploads/2442.pdf.
↵Food and Drug Administration. Letter to John B Cole MD. MARCS-CMS 611902. May 2021. https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/jon-b-cole-md-611902-05052021.
* Department of Health and Human Services Office of Inspector General. The Food and Drug Administration’s oversight of clinical trials. Sep 2007. https://www.oig.hhs.gov/oei/reports/oei-01-06-00160.pdf.
* Food and Drug Administration. Bioresearch monitoring. https://www.fda.gov/media/145858/download.
* FDA takes key action in fight against covid-19 by issuing emergency use authorization for first covid-19 vaccine. Dec 2020. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19.
Original article: https://www.bmj.com/content/375/bmj.n2635
Archive mirrors:
“The [New South Wales Premier] is lying…she’s under an ICAC inquiry that particular lobbyists in Sydney have told her that the ony way she gets out of that inquiry is if she pushes the double-jab…and his clients are AstraZeneca and Pfizer…
Clive Palmer at 9min10sec
…she’s being paid by AstraZeneca and by Pfizer 10’s of millions of dollars to get these policies through to make sure the vaccine is pushed.”
[F]ewer than 1% of vaccine adverse events are reported. Low reporting rates preclude or slow the identification of “problem” drugs and vaccines that endanger public health. New surveillance methods for drug and vaccine adverse effects are needed. Barriers to reporting include a lack of clinician awareness, uncertainty about when and what to report, as well as the burdens of reporting: reporting is not part of clinicians’ usual workflow, takes time, and is duplicative. Proactive, spontaneous, automated adverse event reporting imbedded within EHRs and other information systems has the potential to speed the identification of problems with new drugs and more careful quantification of the risks of older drugs.
One of the UK’s leading childhood health experts has said there is not enough evidence to support vaccinating children against Covid, and the body that will make the decision on whether to jab under-18s has indicated it will take a cautious approach.
Prof Calum Semple, a member of the Scientific Advisory Group for Emergencies (Sage), said there was “rock-solid data” to show that the risk of severe harm to children from Covid was “incredibly low”.
Professor Robert Dingwall said children may be “better protected by natural immunity generated through infection than by asking them to take the ‘possible’ risk of a vaccine”.
…On Wednesday, Prof Dingwall, a social scientist who sits on a subcommittee of the Scientific Advisory Group for Emergencies (Sage) as well as on the JCVI, spoke out, saying the “risk/benefit for teenagers must be firmly established” before any decisions were taken.
In a detailed Twitter thread, he said: “Teenagers are at intrinsically low risk from Covid. Vaccines must be exceptionally safe to beat this. Given the low risk of Covid for most teenagers, it is not immoral to think that they may be better protected by natural immunity generated through infection than by asking them to take the possible risk of a vaccine.”
A Biological Safety Level (BSL 1, 2, 3, or 4) is assigned to a biological lab as a safeguard to protect laboratory personnel, as well as the surrounding environment and community.
With research into potential treatments, therapies and vaccines for the SARS-CoV-2 virus (known widely as the COVID-19 Coronavirus) exploding across the globe, many institutions and laboratories are wondering whether their equipment and lab are considered safe to contain samples of the virus. As discussed in our previous blog on biosafety levels, airborne transmissible diseases like COVID-19 are typically categorized as Biosafety Level 3 (BSL-3). BSL-3 laboratories are almost always purpose-constructed containment laboratories, outfitted with specialized equipment and HVAC systems designed to ensure no airborne particles can exit the contained space.
https://consteril.com/biosafety-level-guidance-covid-19-research/
Dr. Hodkinson, here to discuss the dangers of the COVID-19 vaccines, the possibility of infertility, and the very real concerns about the vaccine-induced spike proteins and what new scientific research is clearly suggesting about their risks to your health.
This guidance is intended for clinical laboratory and support staff who handle or process specimens associated with COVID-19. Guidance for Point-Of-Care Testing can be found here.
All laboratories should perform a site-specific and activity-specific risk assessment and follow Standard Precautions when handling clinical specimens. See Biological Risk Assessment: General Considerations for Laboratories
Refer to List Nexternal icon on the Environmental Protection Agency (EPA) website for EPA-registered disinfectants that have qualified under EPA’s emerging viral pathogens program for use against SARS-CoV-2.
Cultures of SARS-CoV-2 should be handled in a Biosafety Level 3 (BSL-3) laboratory using BSL-3 practices, and inoculation of animals with infectious wild-type SARS-CoV-2 should be conducted in an Animal Biosafety Level 3 (ABSL-3) facility using ABSL-3 practices and respiratory protection.
Suspected and confirmed SARS-CoV-2 positive clinical specimens, cultures, or isolates should be packed and shipped as UN 3373 Biological Substance, Category B.
https://www.cdc.gov/coronavirus/2019-ncov/lab/lab-biosafety-guidelines.html
Published 17 August 2011
Many scientists believe the remarkable properties of graphene could lead to the development of technology such as super-fast computers, flexible mobile phones and even transparent planes among other things. But will the nanomaterial live up to the hype?
…Why all the excitement? When graphite is broken down into graphene, the ultra-thin flakes take on unusual and exciting new properties. Three million of these sheets stacked on top of one another would stand just one millimetre high, and yet graphene is the strongest material ever measured, some 200 times stronger than steel. It is also the most conductive. At the atomic level, it resembles a chicken wire lattice of carbon molecules that is so fine that not even a hydrogen molecule can pass through it.
Top German scientists have found that wearing certain types of face masks for long periods of time could result in potentially hazardous chemicals and harmful microplastics being inhaled deep into human lungs.
Professor Michael Braungart, director at the Hamburg Environmental Institute and co-founder of the world-renowned Cradle to Cradle environmental standard has told Ecotextile News that mask wearers unwittingly run the risk of breathing in carcinogens, allergens and tiny synthetic microfibres by wearing both textile and nonwoven surgical masks for long periods of time.
His recent findings have been backed up by another leading industry textile chemist Dr. Dieter Sedlak, managing director and co-founder of Modern Testing Services Augsburg, Germany in partnership with Modern Testing Services Global, Hong Kong who found elevated concentrations of hazardous fluorocarbons, formaldehyde and other potentially carcinogenic substances on surgical face masks: “I can only say 100 per cent that I have similar concerns to Prof. Braungart.”
We note that a wide range of side effects is being reported following vaccination of previously healthy younger individuals with the gene-based COVID-19 vaccines. Moreover, there have been numerous media reports from around the world of care homes being struck by COVID-19 within days of vaccination of residents. While we recognise that these occurrences might, every one of them, have been unfortunate coincidences, we are concerned that there has been and there continues to be inadequate scrutiny of the possible causes of illness or death under these circumstances, and especially so in the absence of post-mortems examinations.
Last September, Pfizer’s CEO, Albert Bourla, assured the public that ‘we will develop our product, develop our vaccine, using the highest ethical standards’. And the NHS has assured us of the same rigorous standards. So let’s take a look Pfizer’s history of ‘ethical standards’.
In 1992, Pfizer agreed to pay between $165 million and $215 million to settle lawsuits arising from the fracturing of the Bjork-Shiley Convexo-Concave heart valve, which by 2012 had resulted in 663 deaths.
Court approves settlement in Shiley heart-valve case Pfizer Inc. said Wednesday a federal judge has approved the previously announced agreement for settlement of claims to patients with the Bjork-Shiley… upi.com
In 1996, Pfizer conducted an unapproved clinical trial on 200 Nigerian children with its experimental anti-meningitis drug, Trovafloxacin, without parental consent, which led to the death of 11 children from kidney failure and left dozens more disabled. http://news.bbc.co.uk/1/hi/world/africa/6768799.stm …
In 2011, Pfizer paid just $700,000 to four families who had lost a child, and set up a $35 million fund for those disabled by their drug experiment. This cover-up was the basis to the John Le Carré book and film, The Constant Gardener.
Pfizer: Nigeria drug trial victims get compensation US-based pharmaceutical giant Pfizer makes the first compensation payment to Nigerian families affected by a controversial drug trial 15 year… bbc.co.uk
In 2004, Pfizer’s subsidiary, Warner-Lambert, was fined $430 million to resolve criminal charges and civil liabilities for the fraudulent promotion of its epilepsy drug, Neurontin, paying and bribing doctors to prescribe it for uses not approved by the FDA.https://www.justice.gov/archive/opa/pr/2004/May/04_civ_322.htm …
In 2009, Pfizer spent $25.8 million lobbying Congressional lawmakers and federal agencies like the Department of Health and Human Services. Image of government building columns Pfizer Inc Lobbying Profile Pfizer Inc spent $25,819,268 lobbying in 2009. See the details. opensecrets.org
Pfizer’s expenditure on federal lobbying between 2006 and 2014 came to $89.89 million. In 2019 the second largest pharmaceutical company in the world spent $11 million lobbying the US federal government. Image of government building columns Pfizer Inc Lobbying Profile Pfizer Inc spent $11,000,000 lobbying in 2019. See the details. opensecrets.org
In 2009, Pfizer paid the largest health care fraud settlement and criminal fine ever, paying $2.3 billion to avoid criminal and civil liability for fraudulently marketing its anti-inflammatory drug, Bextra, which the FDA had refused due to safety concerns.
Justice Department Announces Largest Health Care Fraud Settlement in American pharmaceutical giant Pfizer Inc. and its subsidiary Pharmacia & Upjohn Company Inc. (hereinafter together “Pfizer”) have agreed to pay $… justice.gov
In 2009, Pfizer paid $750 million to settle 35,000 claims that its diabetes drug, Rezulin, was responsible for 63 deaths and dozens of liver failures. In 1999, an epidemiologist at the FDA said Rezulin was ‘one of the most dangerous drugs on the market’. LA Times logo
Pfizer Agrees to Settle Suit Over Diabetes Drug Rezulin Pfizer Inc. agreed Friday to settle a lawsuit over the diabetes drug Rezulin after a jury earlier in the day awarded $43 million to a Texas woman wh… latimes.com
In 2010, Pfizer was ordered to pay $142.1 million in damages for violating a federal anti-racketeering law by its fraudulent sale and marketing of its epilepsy drug Neurontin for uses not approved by the FDA, including for migraines and bi-polar disorder.
Neurontin Lawsuits – Pfizer Illegal Marketing, Injury Claims Pfizer Marketing Illegal Neurontin. Pfizer Inc. has been ordered to pay $142.1M in damages for violating a federal antiracketeering law in its marketing. yourlawyer.com
In 2010, Pfizer admitted that, in the last 6 months of 2009 alone, it had paid $20 million to 4,500 doctors in the US for consulting and speaking on its behalf, and $15.3 million to 250 academic medical centres for clinical trials.
Pfizer admits paying $35 million to doctors over last 6 months Pfizer among other large pharmaceutical companies recently disclosed payments to doctors and other medical professionals for consulting and … news-medical.net
In 2012, Pfizer paid $45 million to settle charges of bribing doctors and other health-care professionals employed by foreign governments in order to win business. https://www.sec.gov/news/press-release/2012-2012-152htm …
The Chief of the Foreign Corrupt Practices Act Unit said: ‘Pfizer subsidiaries in several countries had bribery so entwined in their sales culture that they offered points and bonus programs to improperly reward foreign officials who proved to be their best customers’.
By 2012, Pfizer had paid $1.226 billion to settle claims by nearly 10,000 women that its hormone replacement therapy drug, Prempro, caused breast cancer.
In 2013, Pfizer agreed to pay $55 million to settle criminal charges of failing to warn patients and doctors about the risks of kidney disease, kidney injury, kidney failure and acute interstitial nephritis caused by its proton pump inhibitor, Protonix.
In 2013, Pfizer set aside $288 million to settle 2,700 claims that its stop-smoking drug, Chantix, caused suicidal thoughts and psychological disorders. The FDA subsequently determined that Chantix is probably associated with a higher risk of heart attack.
In 2013, Pfizer absolved itself of claims that its antidepressant, Effexor, caused congenital heart defects in the children of pregnant woman by arguing that the prescribing obstetrician was responsible for advising the patient about the medication’s use. https://europepmc.org/article/PMC/6424813 …
In 2014, Pfizer paid a further $325 million to settle a lawsuit brought by health-care benefit providers who claimed the company marketed its epilepsy drug, Neurontin, for purposes unapproved by the FDA.
In 2014, Pfizer paid $35 million to settle a law suit accusing its subsidiary of promoting the kidney transplant drug, Rapamune, for unapproved uses, including bribing doctors to prescribe it to patients.
In 2016, Pfizer was fined a record £84.2m for overcharging the NHS for its deregulated anti-epilepsy drug, Phenytoin, by 2,600% (from £2.83 to £67.50 a capsule), increasing the cost to UK taxpayers from £2 million in 2012 to about £50 million in 2013.
In May 2018, Pfizer had 6,000 lawsuits pending against claims that its testosterone replacement therapy products cause strokes, heart attacks, pulmonary embolism and deep vein thrombosis, and were marketed at healthy men for uses not approved by the FDA.
Randomised control trial study showing safety and efficacy of COVID-19 vaccine has clear conflicts of interest.

Many countries across the globe utilized medical and non-medical facemasks as non-pharmaceutical intervention for reducing the transmission and infectivity of coronavirus disease-2019 (COVID-19). Although, scientific evidence supporting facemasks’ efficacy is lacking, adverse physiological, psychological and health effects are established. Is has been hypothesized that facemasks have compromised safety and efficacy profile and should be avoided from use. The current article comprehensively summarizes scientific evidences with respect to wearing facemasks in the COVID-19 era, providing prosper information for public health and decisions making.
…The data suggest that both medical and non-medical facemasks are ineffective to block human-to-human transmission of viral and infectious disease such SARS-CoV-2 and COVID-19, supporting against the usage of facemasks. Wearing facemasks has been demonstrated to have substantial adverse physiological and psychological effects.
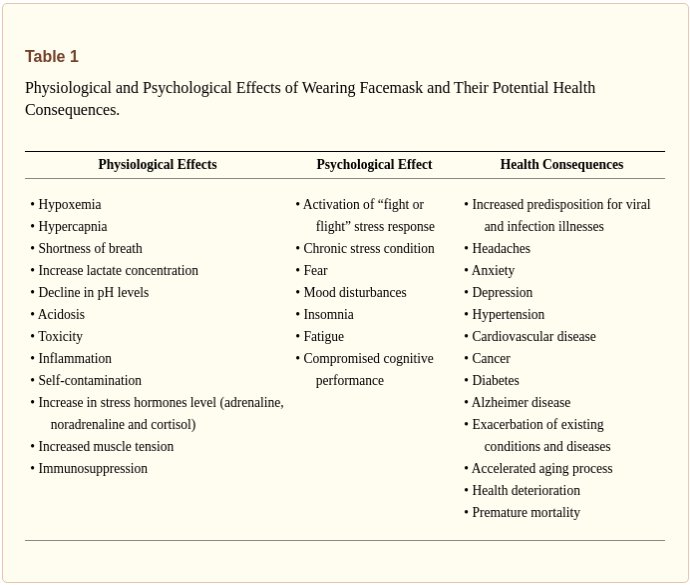
We call for collaborative efforts from scientists, manufacturers, and regulators to assess such risks and look for viable methods to reducing micro(nano)plastics and other respirable debris in face masks and respirators worn by a large population worldwide during the current pandemic.
Products made by diagnostics firm Randox removed from care homes and individuals