It is uncontroversial that all medicines carry the risk of side-effects. As a society, we therefore make a tradeoff between the seriousness of the disease and the risk that a medicine may do some harm. This is the same thought process behind the COVID-19 vaccines. Unfortunately, inaccurate information about the new COVID-19 vaccines have caused a great deal of misinformation to circulate. This page is designed to help you make an informed choice.
Note: The content here was last updated October 2021. For list of vaccines currently authorised in the UK, see the NHS website on the Coronavirus vaccine.
As of early 2023, some mainstream voices who previously championed the new COVID-19 vaccines started talking about serious side-effects. Some commentators say any decision to refuse the vaccine was an uneducated guess. We have archived this page to show that information about potential problems was readily available to all. Choosing to wait or decline entirely was therefore both an evidence-based and rational choice.
Table of contents
- Vaccines authorised for supply in the UK
- Regulations and approval process
- The risk of COVID vs the risk of vaccine side-effects
- What they do and how they work
- Adverse Drug Reactions
- Dr. Mike Yeadon
- Dr. Sucharit Bhakdi
- Dr. Byram Bridle
- Published sources for Adverse Drug Reactions
- Investigations
- Informed consent
- More information
Vaccines authorised for supply in the UK
Three vaccines have been authorised for supply in the UK:
- AstraZeneca COVID 19 Vaccine ChAdOx1
- Moderna COVID-19 vaccine mRNA-1273
- Pfizer COVID-19 mRNA Vaccine BNT162b2
The Patient Information Leaflet (PIL) is the leaflet included with a medicine and should be given before the vaccine is administered in the UK. PILs for the respective vaccines are linked above but if you decide to get the jab, make sure you know which is being given and read through the PIL beforehand.
Countries outside the UK have authorised supply of other vaccines including the Johnson & Johnson, Sinovac and Sputnik candidates.
Regulations and approval process
Parmaceuticals is one of the most heavily regulated industries in the world. Vaccine development, production and supply normally follows a long process of study and oversight from both private and public bodies. This generally lasts at least 10 years but laws are in place that allow the process to be cut short in times of national emergency. According to a research article published on PLOS ONE, “the average vaccine, taken from the preclinical phase, requires a development timeline of 10.71 years and has a market entry probability of 6%.”
Clinical trials
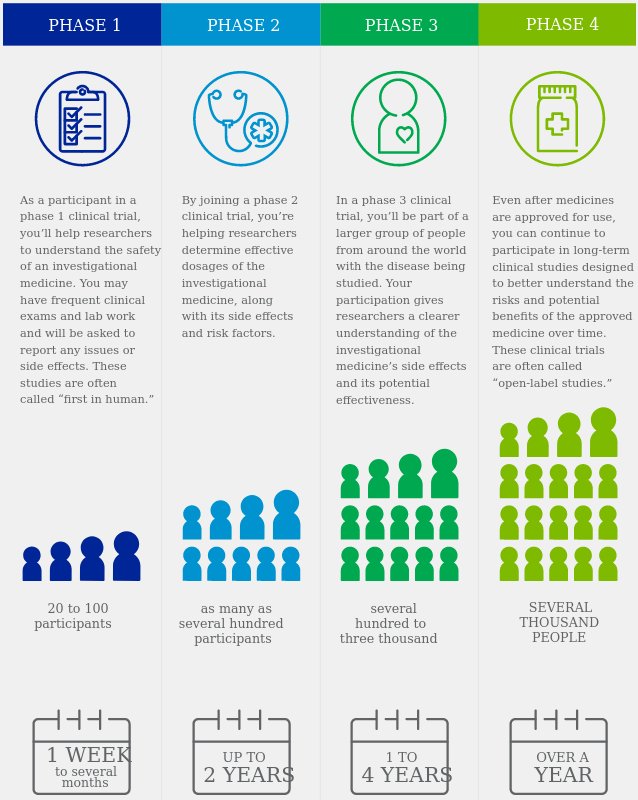
Pfizer’s website has a video and graphic explaining the four phases of clinical trials. As can be seen, under normal conditions, Phase 3 trials last one to four years and are made up of between several hundred to three thousand people.
The History of Vaccines site, from the The College of Physicians of Philadelphia, gives more detail about the clinical trials as they apply to vaccines specifically.
It states that vaccine development generally lasts 10-15 years. The COVID-19 vaccines were deployed to the general population in less than year.
UK Human Medicine Regulations
The UK government approved the supply of the COVID-19 vaccines through temporary authorisation laid out by the Human Medicine Regulations of 2012. The 2012 Regulations were updated on 16 October 2020 to cover:
- temporary authorisations for the COVID-19 vaccines;
- civil liability and immunity for those taking part in the vaccination programmes;
- expanding the healthcare workers who can administer vaccines;
- vaccine promotion;
- an exemption to wholesale licensing requirement.
The vaccines exemption to the licensing requirement means that the vaccines are unlicensed but the manufacturers have immunity from civil liability. AstraZeneca was the first candidate to be given authorisation for emergency supply under in the UK.
The COVID-19 vaccines in the UK are classed as Black Triangle medicines. This means they are subject to more intensive monitoring because of the limited information about their safety.
Emergency use authorization
Vaccine candidates worldwide including AstraZeneca, Johnson & Johnson, Moderna, Pfizer/BioNTech, Sinovac and Sputnik were given emergency use authorization. Authorization was fast-tracked because of the declared emergency.
Anyone taking the vaccines prior to 2023 were therefore part of a clinical trial. The AstraZeneca Phase 3 trial ends in 2023, Moderna Phase 3 trial ends in 2022 and the Pfizer Phase 1/2/3 trial ends in 2023. As of April 2021, the Johnson & Johnson the single-shot vaccine was in the Phase IIa trial.
On 23rd August 2021, The FDA approved the Pfizer-BioNTech COVID-19 vaccine in the US under the brand name Comirnaty. The approval was announced without public consultation, causing drug safety advocates to ask why decisions were being made behind closed doors. Peter Doshi, a senior editor at The BMJ, also questioned the decision, calling for adequate, controlled studies with long term follow up, and the data to be made publicly available. In an interview in September 2021, Dr Robert Malone, inventor of the mRNA platform, criticized the FDA has having conflicts of interest, saying that it is no longer independent from the policy-making apparatus which exists in the Executive Branch of US Government.

Pharmaceutical fraud
Despite heavy regulation, the pharmaceutical industry has repeatedly been involved in civil and criminal violations resulting in heavy damage payments. Pfizer’s settlement for $2.3 billion in 2009 was the largest case of pharmaceutical and health care fraud in US history. AstraZeneca and Johnson & Johnson are also listed among the largest pharmaceutical settlements reached in the US for fraud, off-label promotion and kickbacks.
The media reports that the new COVID-19 vaccines are safe. Nevertheless, in 2020, the British government gave AstraZeneca and Pfizer protection from liability of any damages resulting from their use.
The risk of COVID vs the risk of vaccine side-effects
COVID-19 risk
To assess the risk of the vaccines we first need to ask what are the risks of COVID-19? This has already been established by experts worldwide, such as Professor John Ioannidis of Stanford University.
In at least 80% of cases, the virus produces either no symptoms or a mild cold-like illness. The infection fatality rate for COVID-19 is 0.15%-0.2%. This brings it close to seasonal flu which is around 0.1%-0.2%.
- Children have a greater chance of being struck by lightning than dying of COVID-19.
- Adults are more likely to die in a car accident.
Most of the population have no risk of dying from COVID-19. Studies show that 99.94% survive COVID-19 and will be resistant for a long time. The QCovid risk calculator from Oxford University can be used to calculate your risk of death or hospitalisation.
Vaccine safety
COVID vaccination safety and the risk of adverse drug reactions (ADRs) have yet to be fully studied as they are currently undergoing clinical trials. Vaccine development generally takes 10-15 years and the current vaccines have been released under emergency use authorization only. Vaccine manufacturers AstraZeneca and Pfizer have granted protection from future product liability claims.
See below for emerging evidence of side-effects from the current vaccine rollout.
People in the UK are coming forward to tell of life-changing side-effects after receiving the jab. You can listen to their stories here.
COVID-19 vaccines and children
COVID-19 poses no risk to children, even those in the vulnerable category. The Green Book is the document for public health professionals on immunisation in the UK. As of 7 May 2021, Chapter 14a on COVID-19 states:
SARS-CoV-2 vaccine trials have only just begun in children and there are, therefore, very limited data on safety and immunogenicity in this group. Children and young people have a very low risk of COVID-19, severe disease or death due to SARS-CoV-2 compared to adults and so COVID-19 vaccines are not routinely recommended for children and young people under 16 years of age.
View our guide highlighting the main facts about Children and COVID-19.
Reduction in antibodies
Natural immunity allows for long-term immunity from a range of viruses. This is explained by Dr. Sucharit Bhakdi, Professor Dolores Cahill and Dr. Mike Yeadon in many of their video interviews. Furthermore, researchers at University College London have discovered that some people have natural protection through pre-existing memory T-cells.
However, Dr. David Bauer of the Francis Crick Institute explains that recipients of the Pfizer vaccine have five to six times fewer neutralising antibodies that can recognise and fight the new SARS-CoV-2 Delta variant. These are the antibodies that protect our cells from viruses. that play a key role in protecting us from the Indian variant. Those that have received the Pfizer jabs will therefore be reliant on boosters, possibly for life.
What they do and how they work
The vaccines are not designed to prevent you from being infected or passing on the virus. They are only designed to reduce the symptoms. Dr. Byram Bridle, an Associate Professor on Viral Immunology at the University of Guelph, explains how they work in his talk to New Zealand’s COVID Plan B Group on 12th February 2021.
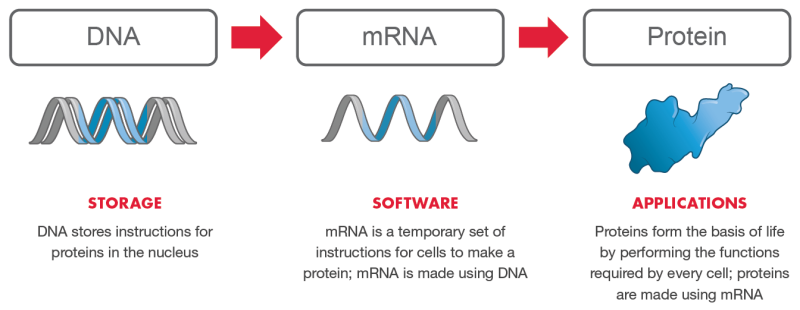
mRNA vaccines
The Moderna and Pfizer/BioNTech technology are forms of messenger RNA (mRNA) vaccines. mRNA medicines are completely new and untested technology. They are designed to be the software platform for the emerging and very lucrative bio-tech industry.
Moderna is quite open about this. On their website, they state that the mRNA Platform is their operating system and the ‘Software of Life’.
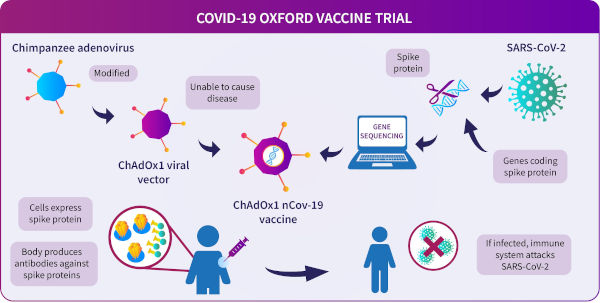
Adenovirus vaccine
Unlike the Pfizer/BioNTech and Moderna coronavirus vaccines, the Oxford/AstraZeneca vaccine is not an mRNA vaccine. It is a viral vector vaccine made from a weakened form of a common cold virus from chimpanzees. The chimpanzee adenovirus is grown in a human cell-line referred to as HEK293 and used to deliver a genetic sequence into the host human cell.
Adverse Drug Reactions
Our archive of articles covering vaccine adverse drug reactions can be found here. Please see below for other investigations and experts warning about adverse drug reactions to the new COVID-19 vaccines.
Dr. Mike Yeadon
Dr. Mike Yeadon, former CSO and VP, Allergy and Respiratory Research Head with Pfizer Global R&D and co-Founder of Ziarco Pharma Ltd, talks about his grave concerns about the Coronavirus jab. Listen to the interview.
Dr. Sucharit Bhakdi
In an April 2021 interview, Dr. Sucharit Bhakdi, a Thai-German microbiologist, discusses mRNA vaccines, blood clots and Cerebral Venous Thrombosis. He warned about the vaccine side-effects months before the roll-out and appears to have been proven correct.
Dr. Byram Bridle
Dr. Byram Bridle, an Associate Professor on Viral Immunology at the University of Guelph, speaks on RADIO 640 Toronto about peer-reviewed studies that possible reasons for side effects such as heart inflammation, VITT (Vaccine induced Thrombosis and Thrombocytopenia) , and other issues may occur in those who have been vaccinated.
Published sources for Adverse Drug Reactions
- UK MHRA Yellow Card Covid-19 Adverse Reactions Data searchable tool compiled by UK Column
- OpenVAERS allows browsing and searching of US VAERS data
- COVID-19 mRNA Pfizer- BioNTech vaccine analysis Report, Run Date: 12-Apr-2021
- COVID-19 vaccine AstraZeneca analysis print Report, Run Date: 12-Apr-2021
- You can register to view the UK Yellow Card Reports of ADRs
- US VAERS Reporting
- Canada Suspected Adverse Reaction
- German Suspected Adverse Reaction
- Europe Suspected Adverse Reaction
- Independent Adverse Drug Reactions Reporting System
- Pfizer Adverse Drug Reactions Reporting System
- AstraZeneca Adverse Drug Reactions Reporting
- NIH begins study of allergic reactions to Moderna, Pfizer-BioNTech COVID-19 vaccines
Investigations
We’re repeatedly told that the COVID vaccination is safe and effective. What does the data show? Independent journalists have been looking into the numbers:
- James Delingpole talks to the NHS medical practice receptionist about adverse reactions to the jab
- UK Column News The Harsh Reality of Vaccine Adverse Effects
- UK Column News No Smoke Without Fire Part 3: Vaccine Adverse Reactions
- UK Column News No Smoke Without Fire Part 2: Interview with former NHS Nurse Debi Evans to discuss Covid 19, Vaccinations and the Immune system
- Ryan Christian Daily Wrap Up, 19 April 2021
- The David Knight Show: Blood Clots an Issue for ALL COVID VACCINES
- COVID-19 adverse drug reaction stories from people in the UK
- Simon Elmer, from Architects for Social Housing, has a three-part series delving into The UK ‘Vaccination’ Programme.
Informed consent
The UK Medical Freedom Alliance, a team of medical professionals, academics, scientists and lawyers, is calling for fully informed consent for all testing, medical treatments and vaccines. They have published a form to support the informed consent process for Covid-19 vaccinations.
Doctors and delegated health care professionals must properly discuss the risks of vaccines with patients, otherwise they are personally responsible for resulting damages. The document protects both the patient and the doctor by presenting a checklist of questions based on General Medical Council guidance on informed consent.
Download the document using the button above which will open a new browser tab from ukmedfreedom.org (333Kb PDF). Print out the form and ensure both parties read and sign the document.
More information
- View the vaccination archives for more articles
- Roadmap for the implementation of actions by the EU
- The four types of COVID-19 vaccine – a snapshot – Healthcare IT News
- Pfizer COVID Vaccine Trial Shows Alarming Evidence of Pathogenic Priming in Older Adults – The Defender