Ministers have agreed a secrecy clause in any dispute with the drugs manufacturer Pfizer over Britain’s Covid vaccine supply. Large portions of the government’s contracts with the company over the supply of 189m vaccine doses have been redacted and any arbitration proceedings will be kept secret.
The revelation comes as Pfizer is accused by a former senior US health official of “war profiteering’’ during the pandemic. In a Channel 4 Dispatches investigation to be broadcast this week, Tom Frieden, who was director of the US Centers for Disease Control and Prevention under Barack Obama, said: “If you’re just focusing on maximising your profits and you’re a vaccine manufacturer … you are war profiteering.”
Pfizer
Browse the articles related to this topic below.
Join our community on Guilded.
Background
Approximately 5.1 million Israelis had been fully immunized against coronavirus disease 2019 (Covid-19) after receiving two doses of the BNT162b2 messenger RNA vaccine (Pfizer–BioNTech) by May 31, 2021. After early reports of myocarditis during adverse events monitoring, the Israeli Ministry of Health initiated active surveillance.
Conclusions
The incidence of myocarditis, although low, increased after the receipt of the BNT162b2 vaccine, particularly after the second dose among young male recipients. The clinical presentation of myocarditis after vaccination was usually mild.
http://archive.today/2021.10.10-032642/https://www.nejm.org/doi/full/10.1056/NEJMoa2109730
A fit and active 26-year-old man has mysterious died with fears he may have suffered fatal side effects 12 days after receiving Pfizer’s Covid vaccine.
Rory James Nairn, 26, had heart palpitations for several days before collapsing to the floor of his Dunedin home in New Zealand on November 17.
The boss of the drugmaker Moderna has warned that Covid-19 vaccines are unlikely to be as effective against the Omicron variant in comments that have added to uncertainty about its impact and unsettled financial markets.
“There is no world, I think, where [the effectiveness] is the same level we had with Delta,” Stéphane Bancel told the Financial Times. “I think it’s going to be a material drop. I just don’t know how much because we need to wait for the data. But all the scientists I’ve talked to … are like, ‘this is not going to be good’.”
A professional musician died after suffering a brain haemorrhage ‘induced by his first dose of the AstraZeneca Covid vaccine’, an inquest has heard.
Matthew Dibble, 40, suffered a ‘catastrophic’ episode just two days after he self-presented at St Thomas’ Hospital in central London complaining of a headache on May 8.
Four months ago, I had my second dose of the Pfizer Covid-19 vaccine. I work for the NHS and fully support Britain’s vaccination campaign, so it was a simple decision for me to make. I had no problems with my first dose and I knew that the vaccines have been found to be highly effective and safe, preventing up to 96 per cent of Covid hospitalisations.
The day after my second dose I began to feel some aches and pains, but I gave little thought to the vaccine and carried on as normal. Four days later though my chest was seriously aching. I tried various stretches and painkillers but my symptoms grew worse. Then there was a sharp pain, piercing into the left side of my chest, near my heart. After a quick call to NHS 111, they sent me directly to Accident & Emergency, where I was strapped up to an electrocardiogram (ECG) machine and given blood tests to see if I was having a heart attack. I’m in my early thirties and relatively healthy, with no family history of heart disease or underlying conditions, and I’d never felt this sensation before.
In the wake of federal vaccine mandates in the U.S., debate has erupted over the waves of fire fighters,police staff, and other workers who have applied for religious exemptions to getting their COVID-19 shots. The number of applications is likely to spike as the January 4 vaccination deadline nears for large private businesses and some healthcare facilities. And one common reason people give for religious exemptions is the link between vaccines and human fetal cells.
It’s true that such cells have been used either in the testing or development and production of COVID-19 vaccines. The cells are grown in a laboratory and were derived from a few elective abortions performed more than three decades ago. These same cell lines are also used to test and advance our understanding of several routine drugs, including acetaminophen, ibuprofen, and aspirin, and they continue to be used for treatment research in diseases such as Alzheimer’s and hypertension.
FDA report shows Pfizer’s clinical trials found 24% higher all-cause mortality rate among the vaccinated compared to placebo group.
The clinical trials of Pfizer’s coronavirus vaccine found that the all-cause mortality rate of the vaccinated group was higher than that of the control group, months after the trials were launched, according to a recently released FDA report.
According to the report, which was released by the US Food and Drug Administration to provide background information on its August 2021 decision to grant full approval for the Pfizer-BioNTech coronavirus vaccine after offering limited emergency authorization of use in last December, six months after the vaccine’s clinical trial began, the total number of deaths reported in the vaccinated group was nearly one-quarter higher than the number of deaths in the placebo group.
http://archive.today/2021.11.19-040650/https://www.israelnationalnews.com/News/News.aspx/317091
Peter Doshi, PhD, is an editor of the BMJ and on the faculty of the University of Maryland.
- Most hopitalisations in the UK are among the fully vaccinated
- Pfizer trials did not show a reduction in deaths–the evidence is flimsy
- These mRNA products are qualitatively different from standard vaccines
- The definitions for vaccines ahve changed
- We shouldn’t assume the new [mRNA products] are like other childhood vaccines which get mandated [in the US]
This is a short clip of the video archive at: https://evidencenotfear.com/vaccine-mandates-expert-panel-highlights-senator-ron-johnson/
Vaccine Mandates Expert Panel Highlights on Senator Ron Johnson’s channel.
In an exclusive and explosive one-hour interview with Veronika Kyrylenko of The New American, pioneering mRNA scientist Dr. Robert Malone explains the intensely corrupt workings of the government regulatory bodies that have mismanaged the pandemic, discusses the problems with the vaccine program and delves into potentially explosive and game-changing revelations about the shady origins of the Covid-19 pandemic in Wuhan, China.
We conclude that the mRNA vacs dramatically increase inflammation on the endothelium and T cell infiltration of cardiac muscle and may account for the observations of increased thrombosis, cardiomyopathy, and other vascular events following vaccination.
Commentary from Dr. Vernon Coleman can be found here.
Introduction
BioNTech Manufacturing GmbH (in partnership with Pfizer Inc.) submitted a Biologics License Application (BLA) STN BL 125742 for licensure of COVID-19 Vaccine, mRNA. The proprietary name of the vaccine is COMIRNATY. COMIRNATY is a vaccine indicated for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 16 years of age and older. The vaccine is administered intramuscularly (IM) as a series of two 30 μg doses (0.3 mL each) 3 weeks apart.
For commentary, see FDA report finds all-cause mortality higher among vaccinated – Israel Nation News.
http://archive.today/2021.10.11-032110/https://www.fda.gov/media/151733/download
In conclusion, the mRNA vacs numerically increase (but not statistically tested) the markers IL-16, Fas, and HGF, all markers previously described by others for denoting inflammation on the endothelium and T cell infiltration of cardiac muscle, in a consecutive series of a single clinic patient population receiving mRNA vaccines without a control group.
https://www.ahajournals.org/doi/10.1161/circ.144.suppl_1.10712
As U.S. health authorities expand use of the leading Covid-19 vaccines, researchers investigating heart-related risks linked to the shots are exploring several emerging theories, including one centered on the spike protein made in response to vaccination.
Researchers aren’t certain why the messenger RNA vaccines, one from Pfizer Inc. and partner BioNTech SE and the other from Moderna Inc., are likely causing the inflammatory heart conditions myocarditis and pericarditis in a small number of cases.
Some theories center on the type of spike protein that a person makes in response to the mRNA vaccines. The mRNA itself or other components of the vaccines, researchers say, could also be setting off certain inflammatory responses in some people.
http://archive.today/2021.11.08-193051/https://www.wsj.com/amp/articles/researchers-probe-link-between-covid-19-vaccines-and-myocarditis-11636290002
On Thursday, the government published its 44th vaccine surveillance report and in a table on page 18 it noted 2,032 deaths of double-vaccinated individuals over 70. More than 3,000 from the same double-jabbed cohort were hospitalised.
Each line in the above table describes “vaccine effectiveness”. That is how much the vaccine REDUCES chances of illness compared to the unvaccinated. The red numbers are NEGATIVE, meaning that vaccine INCREASES chances of geting Covid, compared to the unvaccinated. For example, for vaccinated 40-49 year olds, their chances of getting covid are (see above) 2.28 TIMES HIGHER than the unvaccinated. So their vaccine is a Covid magnet. No kidding.
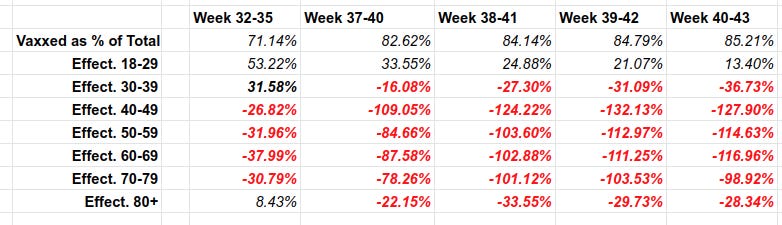
Over 82% of deaths in the UK are among the vaccinated.
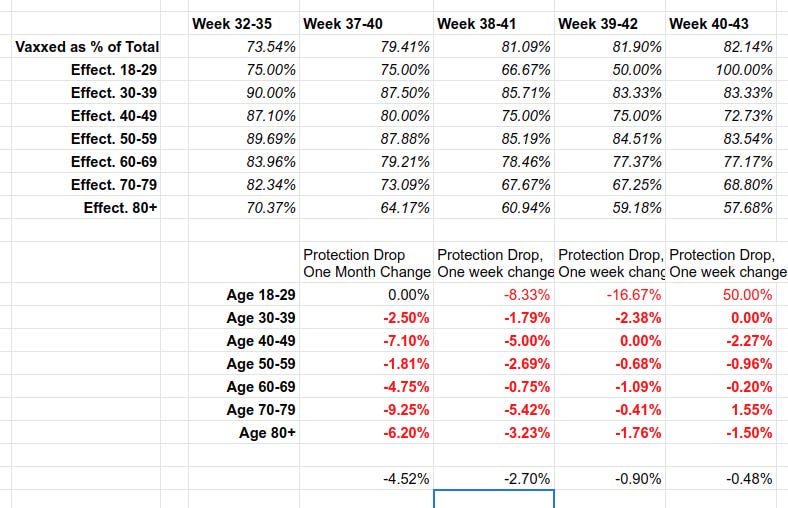
https://igorchudov.substack.com/p/uk-vaccines-weekly-ineffectiveness
Researchers who scoured the records of nearly 800,000 U.S. veterans found that in early March, just as the Delta variant was gaining a toehold across American communities, the three vaccines were roughly equal in their ability to prevent infections.
But over the next six months, that changed dramatically.
By the end of September, Moderna’s two-dose COVID-19 vaccine, measured as 89% effective in March, was only 58% effective.
Revelations of poor practices at a contract research company helping to carry out Pfizer’s pivotal covid-19 vaccine trial raise questions about data integrity and regulatory oversight. Paul D Thacker reports
In autumn 2020 Pfizer’s chairman and chief executive, Albert Bourla, released an open letter to the billions of people around the world who were investing their hopes in a safe and effective covid-19 vaccine to end the pandemic. “As I’ve said before, we are operating at the speed of science,” Bourla wrote, explaining to the public when they could expect a Pfizer vaccine to be authorised in the United States.1
But, for researchers who were testing Pfizer’s vaccine at several sites in Texas during that autumn, speed may have come at the cost of data integrity and patient safety. A regional director who was employed at the research organisation Ventavia Research Group has told The BMJ that the company falsified data, unblinded patients, employed inadequately trained vaccinators, and was slow to follow up on adverse events reported in Pfizer’s pivotal phase III trial. Staff who conducted quality control checks were overwhelmed by the volume of problems they were finding. After repeatedly notifying Ventavia of these problems, the regional director, Brook Jackson, emailed a complaint to the US Food and Drug Administration (FDA). Ventavia fired her later the same day. Jackson has provided The BMJ with dozens of internal company documents, photos, audio recordings, and emails.
Poor laboratory management
On its website Ventavia calls itself the largest privately owned clinical research company in Texas and lists many awards it has won for its contract work.2 But Jackson has told The BMJ that, during the two weeks she was employed at Ventavia in September 2020, she repeatedly informed her superiors of poor laboratory management, patient safety concerns, and data integrity issues. Jackson was a trained clinical trial auditor who previously held a director of operations position and came to Ventavia with more than 15 years’ experience in clinical research coordination and management. Exasperated that Ventavia was not dealing with the problems, Jackson documented several matters late one night, taking photos on her mobile phone. One photo, provided to The BMJ, showed needles discarded in a plastic biohazard bag instead of a sharps container box. Another showed vaccine packaging materials with trial participants’ identification numbers written on them left out in the open, potentially unblinding participants. Ventavia executives later questioned Jackson for taking the photos.
Early and inadvertent unblinding may have occurred on a far wider scale. According to the trial’s design, unblinded staff were responsible for preparing and administering the study drug (Pfizer’s vaccine or a placebo). This was to be done to preserve the blinding of trial participants and all other site staff, including the principal investigator. However, at Ventavia, Jackson told The BMJ that drug assignment confirmation printouts were being left in participants’ charts, accessible to blinded personnel. As a corrective action taken in September, two months into trial recruitment and with around 1000 participants already enrolled, quality assurance checklists were updated with instructions for staff to remove drug assignments from charts.
In a recording of a meeting in late September2020 between Jackson and two directors a Ventavia executive can be heard explaining that the company wasn’t able to quantify the types and number of errors they were finding when examining the trial paperwork for quality control. “In my mind, it’s something new every day,” a Ventavia executive says. “We know that it’s significant.”
Ventavia was not keeping up with data entry queries, shows an email sent by ICON, the contract research organisation with which Pfizer partnered on the trial. ICON reminded Ventavia in a September 2021 email: “The expectation for this study is that all queries are addressed within 24hrs.” ICON then highlighted over 100 outstanding queries older than three days in yellow. Examples included two individuals for which “Subject has reported with Severe symptoms/reactions … Per protocol, subjects experiencing Grade 3 local reactions should be contacted. Please confirm if an UNPLANNED CONTACT was made and update the corresponding form as appropriate.” According to the trial protocol a telephone contact should have occurred “to ascertain further details and determine whether a site visit is clinically indicated.”
Worries over FDA inspection
Documents show that problems had been going on for weeks. In a list of “action items” circulated among Ventavia leaders in early August 2020, shortly after the trial began and before Jackson’s hiring, a Ventavia executive identified three site staff members with whom to “Go over e-diary issue/falsifying data, etc.” One of them was “verbally counseled for changing data and not noting late entry,” a note indicates. At several points during the late September meeting Jackson and the Ventavia executives discussed the possibility of the FDA showing up for an inspection (box 1). “We’re going to get some kind of letter of information at least, when the FDA gets here . . . know it,” an executive stated.
Box 1
A history of lax oversight
When it comes to the FDA and clinical trials, Elizabeth Woeckner, president of Citizens for Responsible Care and Research Incorporated (CIRCARE),3 says the agency’s oversight capacity is severely under-resourced. If the FDA receives a complaint about a clinical trial, she says the agency rarely has the staff available to show up and inspect. And sometimes oversight occurs too late.
In one example CIRCARE and the US consumer advocacy organisation Public Citizen, along with dozens of public health experts, filed a detailed complaint in July 2018 with the FDA about a clinical trial that failed to comply with regulations for the protection of human participants.4 Nine months later, in April 2019, an FDA investigator inspected the clinical site. In May this year the FDA sent the triallist a warning letter that substantiated many of the claims in the complaints. It said, “[I]t appears that you did not adhere to the applicable statutory requirements and FDA regulations governing the conduct of clinical investigations and the protection of human subjects.”5
“There’s just a complete lack of oversight of contract research organisations and independent clinical research facilities,” says Jill Fisher, professor of social medicine at the University of North Carolina School of Medicine and author of Medical Research for Hire: The Political Economy of Pharmaceutical Clinical Trials.
Ventavia and the FDA
A former Ventavia employee told The BMJ that the company was nervous and expecting a federal audit of its Pfizer vaccine trial.
“People working in clinical research are terrified of FDA audits,” Jill Fisher told The BMJ, but added that the agency rarely does anything other than inspect paperwork, usually months after a trial has ended. “I don’t know why they’re so afraid of them,” she said. But she said she was surprised that the agency failed to inspect Ventavia after an employee had filed a complaint. “You would think if there’s a specific and credible complaint that they would have to investigate that,” Fisher said.
In 2007 the Department of Health and Human Services’ Office of the Inspector General released a report on FDA’s oversight of clinical trials conducted between 2000 and 2005. The report found that the FDA inspected only 1% of clinical trial sites.6 Inspections carried out by the FDA’s vaccines and biologics branch have been decreasing in recent years, with just 50 conducted in the 2020 fiscal year.7
The next morning, 25 September 2020, Jackson called the FDA to warn about unsound practices in Pfizer’s clinical trial at Ventavia. She then reported her concerns in an email to the agency. In the afternoon Ventavia fired Jackson—deemed “not a good fit,” according to her separation letter.
Jackson told The BMJ it was the first time she had been fired in her 20 year career in research.
Concerns raised
In her 25 September email to the FDA Jackson wrote that Ventavia had enrolled more than 1000 participants at three sites. The full trial (registered under NCT04368728) enrolled around 44 000 participants across 153 sites that included numerous commercial companies and academic centres. She then listed a dozen concerns she had witnessed, including:
-Participants placed in a hallway after injection and not being monitored by clinical staff
-Lack of timely follow-up of patients who experienced adverse events
-Protocol deviations not being reported
-Vaccines not being stored at proper temperatures
-Mislabelled laboratory specimens, and
-Targeting of Ventavia staff for reporting these types of problems.
Within hours Jackson received an email from the FDA thanking her for her concerns and notifying her that the FDA could not comment on any investigation that might result. A few days later Jackson received a call from an FDA inspector to discuss her report but was told that no further information could be provided. She heard nothing further in relation to her report.
In Pfizer’s briefing document submitted to an FDA advisory committee meeting held on 10 December 2020 to discuss Pfizer’s application for emergency use authorisation of its covid-19 vaccine, the company made no mention of problems at the Ventavia site. The next day the FDA issued the authorisation of the vaccine.8
In August this year, after the full approval of Pfizer’s vaccine, the FDA published a summary of its inspections of the company’s pivotal trial. Nine of the trial’s 153 sites were inspected. Ventavia’s sites were not listed among the nine, and no inspections of sites where adults were recruited took place in the eight months after the December 2020 emergency authorisation. The FDA’s inspection officer noted: “The data integrity and verification portion of the BIMO [bioresearch monitoring] inspections were limited because the study was ongoing, and the data required for verification and comparison were not yet available to the IND [investigational new drug].”
Other employees’ accounts
In recent months Jackson has reconnected with several former Ventavia employees who all left or were fired from the company. One of them was one of the officials who had taken part in the late September meeting. In a text message sent in June the former official apologised, saying that “everything that you complained about was spot on.”
Two former Ventavia employees spoke to The BMJ anonymously for fear of reprisal and loss of job prospects in the tightly knit research community. Both confirmed broad aspects of Jackson’s complaint. One said that she had worked on over four dozen clinical trials in her career, including many large trials, but had never experienced such a “helter skelter” work environment as with Ventavia on Pfizer’s trial.
“I’ve never had to do what they were asking me to do, ever,” she told The BMJ. “It just seemed like something a little different from normal—the things that were allowed and expected.”
She added that during her time at Ventavia the company expected a federal audit but that this never came.
After Jackson left the company problems persisted at Ventavia, this employee said. In several cases Ventavia lacked enough employees to swab all trial participants who reported covid-like symptoms, to test for infection. Laboratory confirmed symptomatic covid-19 was the trial’s primary endpoint, the employee noted. (An FDA review memorandum released in August this year states that across the full trial swabs were not taken from 477 people with suspected cases of symptomatic covid-19.)
“I don’t think it was good clean data,” the employee said of the data Ventavia generated for the Pfizer trial. “It’s a crazy mess.”
A second employee also described an environment at Ventavia unlike any she had experienced in her 20 years doing research. She told The BMJ that, shortly after Ventavia fired Jackson, Pfizer was notified of problems at Ventavia with the vaccine trial and that an audit took place.
Since Jackson reported problems with Ventavia to the FDA in September 2020, Pfizer has hired Ventavia as a research subcontractor on four other vaccine clinical trials (covid-19 vaccine in children and young adults, pregnant women, and a booster dose, as well an RSV vaccine trial; NCT04816643, NCT04754594, NCT04955626, NCT05035212). The advisory committee for the Centers for Disease Control and Prevention is set to discuss the covid-19 paediatric vaccine trial on 2 November.
Footnotes
Provenance and peer review: commissioned; externally peer reviewed.
Competing interests: PDT has been doubly vaccinated with Pfizer’s vaccine.
This article is made freely available for use in accordance with BMJ’s website terms and conditions for the duration of the covid-19 pandemic or until otherwise determined by BMJ. You may use, download and print the article for any lawful, non-commercial purpose (including text and data mining) provided that all copyright notices and trade marks are retained.
https://bmj.com/coronavirus/usage
References
* Bourla A. An open letter from Pfizer chairman and CEO Albert Bourla. Pfizer. https://www.pfizer.com/news/hot-topics/an_open_letter_from_pfizer_chairman_and_ceo_albert_bourla.
* Ventavia. A leading force in clinical research trials. https://www.ventaviaresearch.com/company.
* Citizens for Responsible Care and Research Incorporated (CIRCARE). http://www.circare.org/corp.htm.
* Public Citizen. Letter to Scott Gottlieb and Jerry Menikoff. Jul 2018. https://www.citizen.org/wp-content/uploads/2442.pdf.
↵Food and Drug Administration. Letter to John B Cole MD. MARCS-CMS 611902. May 2021. https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/jon-b-cole-md-611902-05052021.
* Department of Health and Human Services Office of Inspector General. The Food and Drug Administration’s oversight of clinical trials. Sep 2007. https://www.oig.hhs.gov/oei/reports/oei-01-06-00160.pdf.
* Food and Drug Administration. Bioresearch monitoring. https://www.fda.gov/media/145858/download.
* FDA takes key action in fight against covid-19 by issuing emergency use authorization for first covid-19 vaccine. Dec 2020. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19.
Original article: https://www.bmj.com/content/375/bmj.n2635
Archive mirrors:
An article by Toby Rogers, Ph.D.
I was reading the CDC’s “Guidance for Health Economics Studies Presented to the Advisory Committee on Immunization Practices (ACIP), 2019 Update” and I realized that the FDA’s woeful risk-benefit analysis in connection with Pfizer’s EUA application to jab children ages 5 to 11 violates many of the principles of the CDC’s Guidance document. The CDC “Guidance” document describes 21 things that every health economics study in connection with vaccines must do and the FDA risk-benefit analysis violated at least half of them.
Today I want to focus on a single factor: the Number Needed to Vaccinate (NNTV). In four separate places the CDC Guidance document mentions the importance of coming up with a Number Needed to Vaccinate (NNTV). I did not recall seeing an NNTV in the FDA risk-benefit document. So I checked the FDA’s risk-benefit analysis again and sure enough, there was no mention of an NNTV.
Arguments to vaccinate children as young as five against Covid are ‘scientifically weak’, British experts claimed today after the US moved closer to jabbing infants.
…Professor David Livermore, a medical microbiologist at the University of East Anglia told MailOnline: ‘Vaccinating children to protect adults via herd immunity is ethically dubious and is scientifically weak.’
…Professor Russell Viner, a pediatrician and member of the UK Government’s scientific advisory group SAGE, said it was crucial the UK does not ‘rush to a decision’ in the wake of the announcement in the US.