Scientists from the Cleveland Clinic, USA, have recently evaluated the effectiveness of coronavirus disease 2019 COVID-19) vaccination among individuals with or without a history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
The study findings reveal that individuals with previous SARS-CoV-2 infection do not get additional benefits from vaccination, indicating that COVID-19 vaccines should be prioritized to individuals without prior infection. The study is currently available on the medRxiv* preprint server.
Pfizer
Browse the articles related to this topic below.
Join our community on Guilded.
Pfizer-BioNTech announced on Tuesday they have begun late-stage clinical trials of their coronavirus vaccine in children ages five to 11
Lower doses will be used for kids, 10 micrograms, compared to the 30 micrograms that those ages 12 and above receive
The company says it is hoping for data in the latter half of 2021, and is still in the early stages for trials in children between six months to four years old
On Monday, Moderna CEO Stéphane Bancel said he believes his company’s vaccine will be available for kids as young as five years old by early fall
Parents and doctors have been debating about whether or not to inoculate children because they make up just 0.1% of all COVID deaths
Conclusions Individuals who have had SARS-CoV-2 infection are unlikely to benefit from COVID-19 vaccination, and vaccines can be safely prioritized to those who have not been infected before.
https://www.medrxiv.org/content/10.1101/2021.06.01.21258176v2
Dr Bauer of the Francis Crick Institute explains that the Pfizer vaccine produces 5-6 times fewer neutralising antibodies that play a key role in protecting us from the Indian variant. He suggests that booster Pfizer jabs will be essential.
Levels of antibodies in the blood of vaccinated people that are able to recognise and fight the new SARS-CoV-2 Delta variant first discovered in India (B.1.617.2) are on average lower than those against previously circulating variants in the UK, according to new laboratory data from the Francis Crick Institute and the National Institute for Health Research (NIHR) UCLH Biomedical Research Centre, published today (Thursday) as a Research letter in The Lancet.
The results also show that levels of these antibodies are lower with increasing age and that levels decline over time, providing additional evidence in support of plans to deliver a vaccination boost to vulnerable people in the Autumn.
In the case of single-dose recipients, our data show that NAbTs are significantly lower against B.1.617.2 and B.1.351 VOCs relative to B.1.1.7, implying that although a single dose might still afford considerably more protection than no vaccination, single-dose recipients are likely to be less protected against these SARS-CoV-2 variants. These data therefore suggest that the benefits of delaying the second dose, in terms of wider population coverage and increased individual NAbTs after the second dose,7 must now be weighed against decreased efficacy in the short-term, in the context of the spread of B.1.617.2. Worldwide, our data highlight the ongoing need to increase vaccine supply to allow all countries to extend second-dose protection as quickly as possible.
In the longer term, we note that both increased age and time since the second dose of BNT162b2 significantly correlate with decreased NAb activity against B.1.617.2 and B.1.351—both of which are also characteristic of the population in the UK at highest risk of severe COVID-19 (ie, older and vaccinated earlier), independent of other existing factors such as compromised immune status or comorbidity, or geographic-specific responses to vaccination.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)01290-3/fulltext
Accurate information about the development and production of COVID-19 vaccines is essential, especially because many proposed candidates use newer molecular technologies for production of a viral vaccine. One concern regarding the ethical assessment of viral vaccine candidates is the potential use of abortion-derived cell lines in the development, production or testing of a vaccine. This analysis utilizes data from the primary scientific literature when available, along with data from clinical trial documents, reputable vaccine tracking websites, and published commercial information.1 It is the hope that by providing accurate data, recipients can make well-informed decisions regarding vaccine choices.
| Analysis of SARS-CoV-2 (COVID-19) Vaccine Candidates
Last Updated 2 June 2021
|
 DOES NOT USE abortion-derived cell line DOES NOT USE abortion-derived cell line
� Currently undetermined |
||||||
| Sponsor(s)1 | Country | Strategy2 | Clinical Trial Status3 | Public Funding4 | Design & Development | Production | Confirm-atory Lab Tests |
| WHOLE VIRUS VACCINE – LIVE ATTENUATED or INACTIVATED | |||||||
| Beijing Institute of Biological Products/ Sinopharm | China | Inactivated virus
“BBIBP-CorV” Given: Intramuscular 2 doses (3 weeks apart) |
WHO granted Emergency Use Listing (EUL) 7May2021
Early approval in China |

Vero monkey cells Wang et al., Cell 182, P713, 6Aug2020
|

Vero monkey cells Wang et al., Cell 182, P713, 6Aug2020
|

Cytopathic test Vero monkey cells |
|
| Wuhan Institute of Biological Products/ Sinopharm | China | Inactivated virus
“New Crown COVID-19” Given: Intramuscular 2 doses (3 weeks apart) |
Phase 3
Early approval in China |

Vero monkey cells |

Vero monkey cells |
 Plaque reduction neutralization test Vero monkey cells Xia et al., JAMA 324, 951, 13Aug2020 |
|
| Bharat Biotech/Indian Council of Medical Research | India | Inactivated virus “BBV152” Given: Intramuscular 2 doses (2 weeks apart) |
India EUA granted | 
Vero monkey cells |

Vero monkey cells |
 Antibody ELISA Plaque reduction Vero monkey cellsYadav et al., ResearchSquare 10Sept2020 |
|
| Institute of Medical Biology, Chinese Academy of Medical Sciences | China | Inactivated virus “SARS-CoV-2 vaccine” Given: Intramuscular 2 doses (2 weeks apart) |
Phase 3 | 
Vero monkey cells |

Vero monkey cells |
 Antibody ELISA Neutralizing antibody cytopathic effect Vero monkey cells Pu et al., medRxiv, 6Oct2020 Supplement |
|
| John Paul II Medical Research Institute | USA | Live attenuated virus
|
Pre-clinical | 
|

|
� | |
| Research Institute for Biological Safety Problems | Kazakhstan | Inactivated virus
“QazCovid-in” Given: Intramuscular 2 doses (3 weeks apart) |
Phase 3 | � | � | � | |
| Sinovac Biotech Co., Ltd. | China | Inactivated virus
“CoronaVac” Given: Intramuscular 2 doses (2 weeks apart) |
WHO granted Emergency Use Listing (EUL) 1June2021 China granted conditional marketing authorization 8Feb2021 Early approval in China |

Vero monkey cells
|

Vero monkey cells |
 
protein test HEK293 cells |
|
| Valneva and Dynavax | France USA UK |
Inactivated Virus “VLA2001” plus adjuvant CpG1018 Given: Intramuscular 2 doses (3 weeks apart) |
Phase 3 | 
Vero monkey cells
|

Vero monkey cells |
� | |
| VIRAL VECTOR-BASED VACCINE | |||||||
| Altimmune | USA | Replication-deficient
Adenovirus vector “AdCOVID” Given: Intranasal |
Phase 1/2 | 
PER.C6 cells |

PER.C6 cells |
 |
|
| AstraZeneca
University of Oxford
|
USA
UK |
Replication-deficient
Adenovirus vector “AZD1222” “ChAdOX1nCoV-19” Given: Intramuscular 2 doses (4 weeks apart) |
WHO granted Emergency Use Listing (EUL) on 15Feb2021
India EUA granted |
Operation Warp Speed
HHS-BARDA $1.2 Billion CEPI up to $384 Million |

HEK293 cells |

HEK293 cells |
 HEK293 cellsvan Doremalen et al., Nature, 30July2020 MRC-5 cells Almuqrin et al., ResearchSquare 20Oct2020 |
| CanSino Biologics, Inc.
Beijing Institute of Biotechnology, Academy of Military Medical Sciences, PLA of China |
China | Replication-deficient
Adenovirus vector “Ad5-nCoV” Given: Intramuscular 1 dose |
EUA in Chile, Hungary, Pakistan, Mexico | 
HEK293 cells |

HEK293 cells |
 |
|
| Gamaleya Research Institute | Russia | Replication-deficient
Adenovirus vectors (rAd26-S+rAd5-S) “Gam-COVID-Vac” “Sputnik V” Given: Intramuscular 2 doses (3 weeks apart) |
Phase 3
EUA in 39 countries as of Mar2021 Early approval in Russia August 2020 |

HEK293 cells |

HEK293 cells |
 |
|
| ImmunityBio and NantKwest | USA | Replication-deficient Adenovirus vector recombinant “hAd5 S-Fusion + N-ETSD” Given: Subcutaneous |
Phase 1/2 |  E.C7 cells (derivative of HEK293 cells) Rice et al., bioRxiv 30July2020 |

E.C7 cells |
 Protein and antibody tests HEK293T cells Rice et al., bioRxiv 30July2020 Seiling et al., medRxiv 6Nov2020 |
|
| Institut Pasteur and Themis and Merck | USA
France |
Replication-competent recombinant measles virus
“TMV-083” Given: Intramuscular |
Development Discontinued Phase 1/2Phase 1 |
CEPI up to $4.9 Million |  HEK293T HEK293T
Development and rescue of recombinant measles virus “SARS-CoV-2 S-encoding vaccine candidates… were generated as described previously” |
Vero monkey cells |
  Lentiviral vectors for antigenic DCFusogenic testHEK293TFusogenic testS protein expressionVero monkey cellsHörner et al., PNAS 22Dec2020Hörner et al. Supplement |
| Israel Institute for Biological Research (IIBR) | Israel | Replication-competent recombinant vesicular stomatitis virus (VSVΔG) “IIBR-100” Given: Intramuscular1 dose |
Phase 1/2 |  BHK hamster cells Vero monkey cells Yahalom-Ronen et al., bioRxiv 19June2020 |
 Vero monkey cells Yahalom-Ronen et al., bioRxiv 19June2020 |
 Plaque reduction; immunofluorescence Vero monkey cells Yahalom-Ronen et al., bioRxiv 19June2020 |
|
| Janssen Research & Development, Inc.
Johnson & Johnson |
USA | Replication-deficient
Adenovirus vector “Ad26.COV2-S” 1 dose |
FDA Emergency Use Authorization Approved | Operation Warp Speed
HHS-BARDA $1,457,887,081 total |

PER.C6 cells |

PER.C6 cells |
 |
| Laboratorio Avi-Mex | Mexico | Live recombinant Newcastle Disease Virus
Expressing spike-fusion chimeric protein “Patria” Given: Intramuscular or Intranasal |
Phase 1 | 
Bacterial cells BSRT7 hamster cells |
�
Chicken eggs |
�
Neutralization Assay Vero monkey cells |
|
| Meissa Vaccines, Inc. | USA | Live attenuated recombinant RSV viral vector
“MV-014-210” Given: Intranasal 1-3 doses (5 weeks apart) |
Phase 1 |  |

Vero monkey cells Spike expressing, |
� | |
| Rega Institute, KU Leuven | Belgium | Replication-competent attenuated yellow fever vaccine (YF17D) vector
“YF-S0” Given: Intramuscular |
Pre-clinical | 
BHK-21J hamster cells |

BHK-21J hamster cells |
  Antibody titer Pseudovirus HEK293T cells Immunoblot BHK-21J hamster cells Sanchez-Felipe et al., Nature, 1Dec2020 |
|
| ReiThera | Italy | Replication-deficient simian adenovirus encoding S
“GRAd COV2” Given: Intramuscular 1 dose |
Phase 2/3 | 
HEK293T cells Development and rescue of recombinant |

HEK293T cells |

HEK293T cells |
|
| Merck and IAVI | USA | Replication-competent recombinant vesicular stomatitis virus (VSVΔG)
“V590” Given: Intramuscular |
Development Discontinued Phase 1 |
Operation Warp Speed
HHS-BARDA $38,033,570 |

Vero monkey cells |

Vero monkey cells |
� |
| Shenzhen Geno-immune
Medical Institute |
China | Lentivirus minigenes +
Adult human APC (antigen-presenting cells) “COVID-19/aAPC” Given: Subcutaneous 3 doses (2 weeks apart) |
Phase 1 | � |  |
� | |
| Shenzhen Geno-immune
Medical Institute |
China | Lentivirus minigenes +
Adult human CD/T cells (dendritic cells and T cells) “LV-SMENP-DC” Given: Subcutaneous and Intravenous 1 dose |
Phase 1/2 | � |  |
� | |
| Vaxart | USA | Replication-deficient
Adenovirus vector “VXA-CoV2-1” plus dsRNA adjuvant Given: Oral 2 doses (4 weeks apart) |
Phase 1 | 
HEK293 cells |

HEK293 cells |
 |
|
| PROTEIN-BASED VACCINE | |||||||
| Anhui Zhifei Longcom Biopharmaceutical/Institute of Microbiology, Chinese Academy of Sciences | China | Protein vaccine
Recombinant RBD dimer plus adjuvant ”ZF2001” Given: Intramuscular 2 or 3 doses (28 days apart) |
Phase 3 | 
HEK293T cells |

CHO hamster cells |

Pseudovirus HEK293T cells |
|
| Clover Biopharmaceuticals, Inc. | China | Protein vaccine
“SCB-2019” plus adjuvant CpG 1018 Given: Intramuscular |
Phase 2/3 | CEPI up to $69.5 Million |  cDNA in expression vector; transfect CHO hamster cells cDNA in expression vector; transfect CHO hamster cells
|

CHO hamster cells |
  PseudovirusHEK293 cellsRef’d: Nie et al., Emerging Microbes & Infections 24Mar2020Cytopathic effect Vero monkey cellsLiang et al., bioRxiv, 24Sept2020 |
| COVAXX and United Biomedical | USA Taiwan |
Protein vaccine
“UB-612” S1-RBD-protein; Multitope Peptide-Based Vaccine (MVP) Given: Intramuscular |
Phase 2/3 |  cDNA in expression vector; transfect CHO hamster cells cDNA in expression vector; transfect CHO hamster cells
|

CHO hamster cells |
  Antibody blocked binding to hACE2 HEK293 Guirakhoo et al., bioRxiv, 30Nov2020 |
|
| Federal Budgetary Research Institution State Research Center of Virology and Biotechnology “Vektor” | Russia | Protein vaccine “EpiVacCorona” chemically synthesized peptide antigens of SARS-CoV-2, conjugated to a carrier protein adsorbed on an aluminum-containing adjuvant Given: Intramuscular2 doses (3 weeks apart) |
Phase 3
Early approval in Russia Oct 2020 |
� | 
chemically synthesized peptide antigens |
� | |
| Instituto Finlay de Vacunas | Cuba | Protein vaccine “Finlay-FR-1” (“Soberana 01”) Receptor-binding domain (RBD) SARS-CoV-2 spike + adjuvant Given: Intramuscular2 doses (4 weeks apart) |
Phase 1/2 Phase 1 |
� RBD produced in mammalian cells Garcia-Rivera, MEDICC Review, 30Oct2020 |
� RBD produced in mammalian cells Garcia-Rivera, MEDICC Review, 30Oct2020 |
� | |
| Instituto Finlay de Vacunas | Cuba | Protein vaccine “Finlay-FR-2” (“Soberana 02”) Receptor-binding domain (RBD) SARS-CoV-2 spike chemically bound tetanus toxoid + adjuvant Given: Intramuscular2 doses (4 weeks apart) |
Phase 2 Phase 1 |
� RBD produced in mammalian cells Garcia-Rivera, MEDICC Review, 30Oct2020 |
� RBD produced in mammalian cells Garcia-Rivera, MEDICC Review, 30Oct2020 |
� | |
| John Paul II Medical Research Institute | USA | Recombinant Protein
Perinatal human cells (term umbilical cord and placental) |
Pre-clinical | 
|

|
� | |
| Kentucky BioProcessing, Inc. (British American Tobacco) |
USA | Protein vaccine “KBP-201” Plant-expressed RBD Given: Intramuscular2 doses (3 weeks apart) |
Phase 1/2 | 
Recombinant DNA sequence for RBD of SARS-CoV-2 |

Plant expression of RBD peptide |
� | |
| Medicago | Canada | Protein on Virus-Like Particle
“CoVLP” Plant-expressed spike protein particle with adjuvant, CpG1018 or AS03 Given: Intramuscular 2 doses (3 weeks apart) |
Phase 2/3 | 
Recombinant DNA sequence in Agrobacterium, transformation of plant cells |

Plant expression of protein and VLP |
 
Pseudovirus HEK293 cells |
|
| Migal Galilee Research Institute | Israel | Protein vaccine
E. coli expressed chimeric S and N proteins Given: Oral |
Pre-clinical | � | 
Bacterial production system |
� | |
| Novavax | USA | Protein vaccine
“NVX-CoV2373” Baculovirus expression plus Matrix M adjuvant Given: Intramuscular 2 doses (3 weeks apart) |
Phase 3 | Operation Warp Speed
HHS-BARDA $1,600,434,523 CEPI up to $388 Million |
 |

Sf9 insect cells |
 
Pseudovirus HEK293 cells |
| Sanofi and GSK
Protein Sciences
|
USA
France |
Protein vaccine
Baculovirus expression plus AS03 adjuvant Given: Intramuscular 2 doses (3 weeks apart) |
Phase 3 | Operation Warp Speed
HHS-BARDA $2,072,775,336 total |

Recombinant baculovirus |

Sf9 insect cells |
 
Pseudovirus HEK293T cells |
| Sorrento | USA | Protein vaccine
“T-VIVA-19” SARS-Cov-2 spike protein S1 domain fused with human IgG-Fc Given: Intramuscular |
Pre-clinical |  |

CHO cells |

Antibody ELISA; Neutralization assays Vero monkey cells |
|
| Sorrento | USA | Protein vaccine
“STI-6991” SARS-Cov-2 spike protein expressed on K562 cells |
Pre-clinical | � | 
K562 cells |
� | |
| University of Pittsburgh | USA | Protein vaccine
Adenovirus-expressed recombinant proteins “PittCoVacc” Given: Microneedle arrays |
Pre-clinical | 
HEK293 cells |

HEK293 cells |
 |
|
| University of Queensland and CSL Ltd. | Australia | Protein vaccine
“V451” Recombinant protein with proprietary molecular clamp Given: Intramuscular |
HALTED | CEPI up to $4.5 Million |  |

expiCHO hamster cells
|
� |
| Walter Reed Army Institute of Research (WRAIR) / U.S. Army Medical Research and Development Command | USA | Protein vaccine
”SpFN” Spike-Ferritin nanoparticle with ALFQ adjuvant Given: Intramuscular 2-3 doses (4 weeks apart; plus 6 months after initial injection) |
Phase 1 | 
Expi293 cells |

Expi293 cells |
 
Pseudovirus HEK293 cells Virus neutralization Vero monkey cells |
|
| RNA VACCINE | |||||||
| Arcturus Therapeutics | USA | mRNA vaccine
self-transcribing, replicating “LUNAR-CoV19” (“ARCT-021”) in vitro transcription reaction with T7 RNA polymerase from STARR plasmid template LUNAR proprietary lipid nanoparticle encapsulated Given: Intramuscular 1 dose |
Phase 2
|

Sequence designed on computer |

No cells used |
 
protein test HEK293 Protein expression Hep3b cells Plaque reduction neutralization Vero monkey cells |
|
| CureVac | Germany | mRNA vaccine
non-replicating “CVnCoV” in vitro transcription lipid nanoparticle encapsulated Given: Intramuscular 2 doses (4 weeks apart) |
Phase 3 | CEPI up to $15.3 Million | 
Sequence designed on computer |

No cells used |

Protein test Reticulocyte lysate, |
| Imperial College London | UK | mRNA vaccine
Self-amplifying ”LNP-nCoVsaRNA” in vitro transcription lipid nanoparticle encapsulated Given: Intramuscular 2 doses |
Phase 1 | 
Expression plasmid HEK293 cells |

No cells used |
 
Pseudovirus HEK293T cells |
|
| Moderna, Inc.
with National Institutes of Health |
USA | mRNA vaccine
non-replicating “mRNA-1273” T7 RNA polymerase-mediated transcription from DNA plasmid template LNP (lipid nanoparticle) encapsulated Given: Intramuscular 2 doses (4 weeks apart) |
FDA Emergency Use Authorization Approved | Operation Warp Speed
HHS-BARDA $2,479,894,979 total CEPI up to $1 Million |
Sequence designed on computer |

No cells used |
 
protein test & pseudovirus HEK293 cells Plaque reduction neutralization Vero monkey cells |
| Pfizer and BioNTech | USA
Germany |
mRNA vaccine
non-replicating “BNT-162a1,b1,b2,b3,c2” nucleoside-modified mRNA in vitro transcribed by T7 polymerase from a plasmid DNA template LNP (lipid nanoparticle) encapsulated Given: Intramuscular 2 doses (3 weeks apart) |
FDA Emergency Use Authorization Approved
UK EUA granted
|
Operation Warp Speed
HHS-BARDA $1.95 Billion |

Sequence designed on computer |

No cells used |
 
protein test & pseudovirus HEK293 cells Neutralization assay Vero monkey cells |
| Providence Therapeutics | Canada | mRNA vaccine
“PTX-COVID19-B” mRNA in vitro transcription from plasmid template using T7 RNA polymerase Given: Intramuscular 2 doses (4 weeks apart) |
Phase 1 | 
HEK293T cells used to select mRNA candidate |

No cells used |
 
Pseudovirus, serum neutralization HEK293T cells Vero monkey cells |
|
| Sanofi Pasteur and
Translate Bio |
USA
France |
mRNA vaccine
non-replicating “MRT5500” synthesized by in vitro transcription employing RNA polymerase with a plasmid DNA template LNP (lipid nanoparticle) encapsulated Given: Intramuscular 2 doses (3 weeks apart) |
Phase 1/2 | 
HEK293T cells used to select mRNA candidate |

No cells used Kalnin et al., npj Vaccines 19Apr2021 |
 
protein test & pseudovirus HEK293 cells |
|
| DNA VACCINE | |||||||
| Genexine | Korea | DNA vaccine
“GX-19” DNA synthesized in vitro, placed in plasmid vector Given: Intramuscular and Electroporation 2 doses (4 weeks apart) |
Phase 1/2 | 
Sequence designed on computer |

No cells used |
 No cells used No cells used
|
|
| Inovio Pharmaceuticals | USA | DNA vaccine
“INO-4800” DNA synthesized in vitro, placed in plasmid vector Given: Intradermal Electroporation 2 doses (4 weeks apart) |
Phase 2/3 | Operation Warp Speed
CEPI up to $22.5 Million |

Sequence designed on computer |

No cells used |
 
protein test & pseudovirus HEK293 cells |
| Osaka University, AnGes, Takara Bio | Japan | DNA vaccine
“AG0301-COVID19” 2 doses (2 weeks apart) |
Phase 2/3 | 
Sequence designed on computer |

No cells used |

Virus neutralization Vero E6 cells monkey cells |
|
| Symvivo Corporation | Canada | DNA vaccine
“bacTRL-spike” Given: Oral, bacteria bind to gut lining 1 dose |
Phase 1 | � | 
No cells used |
� | |
| Zydus Cadila | India | DNA vaccine
“ZyCov-D” 3 doses (4 weeks apart) |
Phase 3 | 
Sequence designed on computer |

No eukaryotic cells used |

Expression analysis |
|
Fresh from their smash reunion tour this weekend, James and Toby recap the weekend’s anti-lockdown march that wound from Parliament Square to Toby’s doorstep.
We then parse the testimony of the PM’s former “top man,” Dominic Cummings, before the House’s Health and Science select committees last week and who came out of it better. Do you want a Prime Minister or a Monarch (and by “monarch” we’re not talking about the one we’ve already got.)
https://ricochet.com/podcast/london-calling/release-us-from-this-servitude/
CDC looking into reports that a small number of teens and young adults vaccinated against the coronavirus that may have experienced heart problems
Condition, known as myocarditis, results in an inflammation of the heart muscle which can occur following certain infections
Problems have been occurring four days after the second dose has been given
Dozens of cases have been reported to the agency in recent week
It is not yet clear which vaccine might be responsible, Moderna or Pfizer
The agency’s vaccine safety group was sparse in details, saying only that there were ‘relatively few’ cases and levels were similar to normal
Group also said that the conditions may be entirely unrelated to vaccination
The process of learning about and developing an investigational medicine is divided into four phases. At first, very few people receive the medicine being studied. The number of people participating in clinical studies grows along with our understanding of the investigational medicine, and the research continues as long as the potential benefits outweigh the risks.
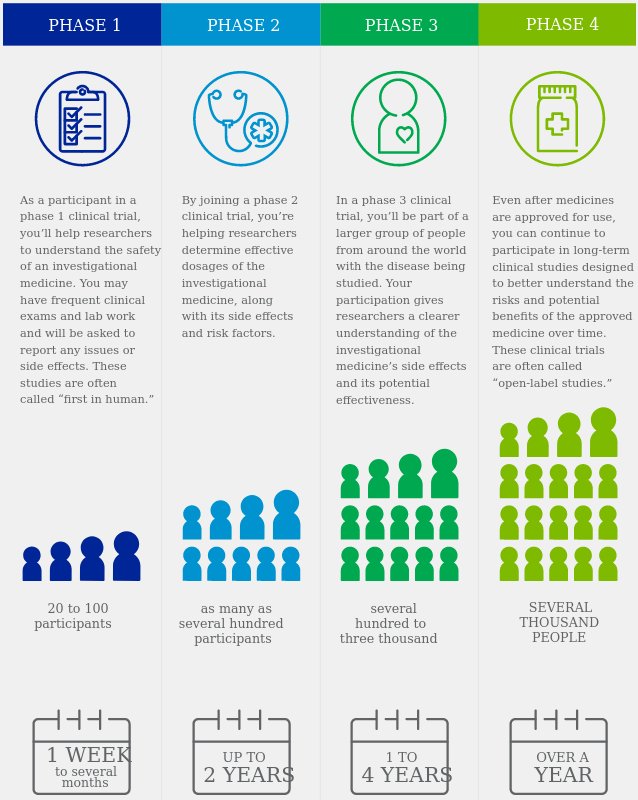
https://www.pfizer.com/science/clinical-trials/guide-to-clinical-trials/phases
Hard to justify right now for most children in most countries
Following widespread vaccination against SARS-CoV-2 of older adults and other highly vulnerable groups, some high income countries are now considering vaccinating children; just days ago, the US Food and Drug Administration authorized the use of the Pfizer/BioNTech vaccine in children 12-15 years of age. Young people have been largely spared from severe covid-19 so far, and the value of childhood vaccination against respiratory viruses in general remains an open question for three reasons: the limited benefits of protection in age groups that experience only mild disease; the limited effects on transmission because of the range of antigenic types and waning vaccine induced immunity; and the possibility of unintended consequences related to differences in vaccine induced and infection induced immunity. We discuss each in turn.
So, the third wave is officially no more. New modelling by SPI-M, the government’s committee on modelling for pandemics, has, at a stroke, eradicated the predicted surge in new infections, hospital admissions and deaths which it had pencilled in for the autumn or winter as a result of lockdown being eased.
…As Philip Thomas explained here on Sunday, Imperial College has also assumed strangely low estimates for the number of people in Britain carrying antibodies. If you are going to use assumptions that are far more pessimistic than real world data suggests, it is small wonder that SPI-M keeps predicting waves and surges that turn out to be wide of the mark. The question is: why are these modelling teams using such negative assumptions?
https://www.spectator.co.uk/article/how-likely-is-a-third-wave-
The vaccine brought in $3.5 billion in revenue in the first three months of this year, nearly a quarter of its total revenue, Pfizer reported. The vaccine was, far and away, Pfizer’s biggest source of revenue.
On Tuesday, the company announced just how much money the shot is generating.
The vaccine brought in $3.5 billion in revenue in the first three months of this year, nearly a quarter of its total revenue, Pfizer reported. The vaccine was, far and away, Pfizer’s biggest source of revenue.
The company did not disclose the profits it derived from the vaccine, but it reiterated its previous prediction that its profit margins on the vaccine would be in the high 20 percent range. That would translate into roughly $900 million in pretax vaccine profits in the first quarter.
https://www.nytimes.com/2021/05/04/business/pfizer-covid-vaccine-profits.html
I won’t have been the only parent concerned by news last week that the Pfizer vaccine may be approved for use on children as early as June and potentially rolled out to school pupils from September. Healthy children are at almost no serious risk from Covid-19 – the recovery rate for this age group has been calculated at over 99.99 per cent. The argument that children should have the vaccine is not based on a belief that they need or benefit from it but on the logic that it would be good for our communities at large if children were jabbed. In short, those advocating it assume that children have an obligation to protect adults.
It’s worth noting that the UK Government has granted immunity from liability for harms to all Covid-19 vaccine manufacturers. Can we really ask children to accept a greater risk than the manufacturers themselves are prepared to live with?
https://www.telegraph.co.uk/news/2021/05/03/healthy-children-simply-do-not-need-covid-jab
Danish authorities have opted for a more cautious path, even though Reuters reported that excluding J&J’s shot could significantly delay the country’s vaccination efforts.
Danish drug officials last month abandoned the use of AstraZeneca’s Covid-19 vaccine, also citing the risk of blood clots. In March, Denmark became the first country in the world to temporarily suspend the AstraZeneca shot, but unlike its European neighbors, the country made that suspension permanent.
https://www.rt.com/news/522790-denmark-cancels-johnson-vaccine/
Schools back mass vaccinations for children, with headteachers saying that “peer pressure” will boost take up.
Education leaders would be willing to help facilitate a vaccine roll-out at schools around the country, according to Geoff Barton, general secretary of the Association of School and College Leaders (ASCL), the largest union for secondary school heads.
…“I think there will be a sense of schools wanting to step up and play their part and explain to children why having the vaccine is important during assemblies and in tutor time.”
…He explained that vaccinating children at school could result in higher take-up because pupils would not want to feel socially isolated by refusing to have the jab.
…“The peer pressure of seeing that your friends are lining up to do it is likely to make the overall numbers taking up the vaccine higher,” he said. Some scientists have argued that if Covid rates rose significantly it would be a priority to vaccinate children to prevent any more disruption or closures of schools during the next academic year.
Published 19 August 1992
New York-based Pfizer said the agreement will cost it between $165 million and $215 million, on a pretax basis, and will be offset by proceeds from the sale of most of its Shiley Inc. assets earlier this year as well as expected insurance reimbursements.
Under-40s may be offered an alternative to the Oxford-AstraZeneca vaccine after blood clot reports doubled, reports claim.
The chance of dying from a blood clot after having the jab is about one in one million – with 19 fatalities from around 20 million vaccinations. However, the total number of people in the UK who developed blood clots after getting one dose has gone from 79 to 168 in a fortnight, Medical Healthcare Products and Regulatory Agency (MHRA) data suggests.
Many of the vaccines developed to protect against COVID-19 are forms of messenger RNA (mRNA) vaccines.
The Moderna and Pfizer/BioNTech vaccines are forms of mRNA vaccine.
Unlike the Pfizer/BioNTech and Moderna coronavirus vaccines, the Oxford/AstraZeneca vaccine is not an mRNA vaccine.
Instead, the AstraZeneca vaccine is a viral vector vaccine made from a weakened form of a common cold virus from chimpanzees.
Robin Hauser, a pediatrician in Tampa, Florida, got COVID in February. What separates her from the vast majority of the tens of millions of other Americans who have come down with the virus is this: She got sick seven weeks after her second dose of the Pfizer-BioNTech vaccine.
https://www.pbs.org/newshour/health/the-shock-and-reality-of-catching-covid-after-being-vaccinated