Under-40s may be offered an alternative to the Oxford-AstraZeneca vaccine after blood clot reports doubled, reports claim.
The chance of dying from a blood clot after having the jab is about one in one million – with 19 fatalities from around 20 million vaccinations. However, the total number of people in the UK who developed blood clots after getting one dose has gone from 79 to 168 in a fortnight, Medical Healthcare Products and Regulatory Agency (MHRA) data suggests.
Adverse Drug Reaction
Browse the articles related to this topic below.
Join our community on Guilded.
A mother has claimed her legs erupted into painful blood-filled blisters that ‘merged together’ after receiving AstraZeneca’s coronavirus vaccine.
Sarah Beuckmann, from Glasgow, said she suffered flu-like symptoms after getting her first dose in mid-March — a very common side-effect. But the 34-year-old began to feel a tingling sensation in her legs just a week later and noticed a rash flaring up around her ankles.

Dr. Sucharit Bhakdi, a Thai-German microbiologist, discusses mRNA vaccines, blood clots and Cerebral Venous Thrombosis. He warned about the vaccine side-effects months before the roll-out and appears to have been proven correct.
- Luke Garrett, 20, died after suffering a seizure in Tarbolton, South Ayrshire
- Mr Garrett had muscular dystrophy and had been shielding for around a year
- But he died in the early hours of the morning the day after getting the jab
- Mother Tricia Garrett, 49, believes son would still be alive if he didn’t get the jab
- MHRA are investigating his death but there’s no evidence of jab causing seizures
All UK spontaneous reports received between 04/01/21 and 05/04/21 for COVID-19 vaccine Oxford University/AstraZeneca
All UK spontaneous reports received between 9/12/20 and 05/04/21 for COVID-19 vaccine mRNA Pfizer/BioNTech vaccine analysis print
- Beckie Wilson from Stoke-on-Trent reported experiencing a shortness of breath
- She’s now been put on blood thinning medications and is off work for 3 weeks
- Vaccine advisers now recommend healthy under-30s be given alternative jab
A Covid vaccination site shuttered its doors early after 13 patients suffered bad reactions to their shots. The mass vaccination event at Dick’s Sporting Goods Part in Commerce Field, Colorado, was halted on Wednesday, with two patients taken to hospital for further evaluation.
Dr Mike Yeadon, former CSO and VP, Allergy and Respiratory Research Head with Pfizer Global R&D and co-Founder of Ziarco Pharma Ltd, talks about his grave concerns about the Coronavirus jab.
In conclusion, the observed safety profile is considered favourable. Longer term safety data is awaited
from the ongoing clinical trials.
There are very limited data on the use of the vaccine in immunocompromised individuals and on use in pregnancy and breastfeeding. No data was generated with mRNA-1273 when administered concomitantly with other vaccines.
The CHMP considers the following measures necessary to address the missing safety data in the
context of a conditional MA:The final clinical study report will be submitted no later than December 2022 and is subject to a specific obligation laid down in the MA. This will provide long-term data.
Reference is made to the Request for Comments and Advice submitted 04 February 2021 regarding Pfizer/BioNTech’s proposal for the clinical and post-authorization safety data package for the Biologics License Application (BLA) for our investigational COVID-19 Vaccine (BNT162b2). Further reference is made to the Agency’s 09 March 2021 response to this request, and specifically, the following request from the Agency.
The download link is available from Public Health and Medical Professionals for Transparency.
Important points from this report include:
- A seven-year-old experienced a stroke.
- One child and one infant suffered facial paralysis.
- One infant had a kidney adverse event, either kidney injury or failure.
- Of the 34 cases, 24 (71%) were classified as serious.
- Predominantly female patients were affected — at least 25 of 34 (73.5%) patients.
- Table 6 reports 34 cases of use in pediatric individuals. However, 28 additional cases were excluded because details such as height and weight were “not consistent with pediatric subjects.”
- Ages ranged from two months to nine years, with median 4.0 years, which means half the children were under four years of age.
- 132 adverse events were reported in the 34 children – i.e., an average of 3.88 AEs per child.
Summary from Daily Clout journalists:
“We fully support the swine flu vaccination programme … The vaccine has been thoroughly tested,” they declared in a joint statement.
Except, it hadn’t. Anticipating a severe influenza pandemic, governments around the world had made various logistical and legal arrangements to shorten the time between recognition of a pandemic virus and the production of a vaccine and administration of that vaccine in the population. In Europe, one element of those plans was an agreement to grant licences to pandemic vaccines based on data from pre-pandemic “mock-up” vaccines produced using a different virus (H5N1 influenza). Another element, adopted by countries such as Canada, the US, UK, France, and Germany, was to provide vaccine manufacturers indemnity from liability for wrongdoing, thereby reducing the risk of a lawsuit stemming from vaccine related injury.
https://web.archive.org/web/20200408051329/https://www.bmj.com/content/362/bmj.k3948
TED (Tenders Electronic Daily) is the online version of the ‘Supplement to the Official Journal’ of the EU, dedicated to European public procurement.
The MHRA urgently seeks an Artificial Intelligence (AI) software tool to process the expected high volume of Covid-19 vaccine Adverse Drug Reaction (ADRs) and ensure that no details from the ADRs’ reaction text are missed.

https://ted.europa.eu/udl?uri=TED:NOTICE:506291-2020:TEXT:EN:HTML&tabId=1
- In July Health Secretary Matt Hancock claimed that conspiracy theorists are putting lives at risk
- The UK government’s Vaccine Damage Payment scheme is proof that vaccines can be unsafe
- Eligibility criteria Vaccine Damage Payment changed in 2015
- Update October 2020: AstraZeneca protected from vaccine liability
- Update November 2020: MHRA expects high volume of COVID-19 vaccine adverse drug reaction
- Update December 2020: Pfizer is given protection from legal action by the UK government
Discussion around vaccinations can be very contentious. There’s great nuance in this area and a short post will not do justice to the complex issues surrounding the usefulness and safety of vaccines. Nevertheless, while vaccines may have their role in protecting target populations from disease, not all have been proven safe to an acceptable level as shown in the resources below.
The UK government’s Vaccine Damage Payment scheme is probably the strongest proof that vaccines can be unsafe. Under the Vaccine Damage Payment scheme, people who have been severely disabled as a result of a vaccination against certain diseases can be eligible for a one-off tax-free payment of £120,000.
Conspiracy theorists are putting lives at risk?
It is an objective fact that a compensation scheme exists for those who have been damaged by vaccines. Nevertheless, Health Secretary Matt Hancock claimed that conspiracy theorists are putting lives at risk:
“Those who promulgate lies about dangers of vaccines that are safe and have been approved–they are threatening lives…”
Source: The Independent, 20 July 2020
Clearly, concerns about the safely of vaccines cannot be lies if there is a vaccine damage compensation scheme in place.
Eligibility changed in 2015
Eligibility requirements for vaccines covering certain diseases are listed and change over time. Interestingly, sometime around 2015, damage from vaccines for influenza caused by pandemics are explicitly listed as not eligible.
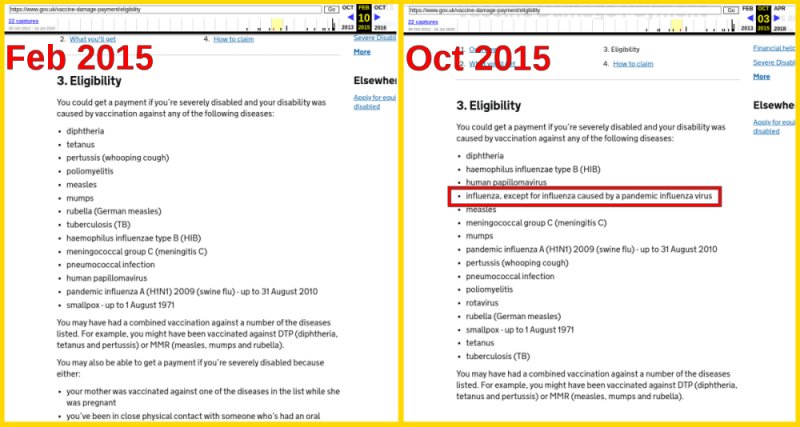
We do not know how the government compiles is eligibility criteria or why this change was made. However, it would be worthwhile to keep an eye on this list to see if the status of the upcoming COVID-19 vaccines.
AstraZeneca protected from vaccine liability
Update 1 August 2020: On 30 July 2020, Reuters reported that AstraZeneca, the UK government’s partner for developing its COVID-19 vaccine, will be exempt from coronavirus vaccine liability claims in most countries. The countries have not been named but Ruud Dobber, a member of Astra’s senior executive team, commented:
“This is a unique situation where we as a company simply cannot take the risk if in … four years the vaccine is showing side effects.
In the contracts we have in place, we are asking for indemnification. For most countries it is acceptable to take that risk on their shoulders because it is in their national interest.”
MHRA expects high volume of COVID-19 vaccine adverse drug reaction
Update November 2020: It came to light in mid-November that the UK’s Medicines & Healthcare products Regulatory Agency (MHRA) put out a contract award notice for an Artificial Intelligence (AI) software tool. It appears they expect a high volume of COVID-19 vaccine Adverse Drug Reaction (ADRs) from the upcoming vaccines:
…it is not possible to retrofit the MHRA’s legacy systems to handle the volume of ADRs that will be generated by a Covid-19 vaccine. Therefore, if the MHRA does not implement the AI tool, it will be unable to process these ADRs effectively.
Pfizer given legal indemnity
Update 2 December 2020: According to the Independent, Pfizer now has a legal indemnity from being sued by patients who develop any complications from its new mRNA vaccine that will be rolled out in the UK. NHS staff providing the vaccine will also be protected.
Resources
- UK Government Vaccine Damage Payment (gov.uk)
- Ministers lose fight to stop payouts over swine flu jab narcolepsy cases (The Guardian)
- Dengue vaccine fiasco leads to criminal charges for researcher in the Philippines (Science Magazine)
- Polio outbreaks in Africa caused by mutation of strain in vaccine (The Guardian)
- Pakistan accused of cover-up over fresh polio outbreak – (The Guardian)
- The Vaccination Debate (The Guardian)
- AstraZeneca to be exempt from coronavirus vaccine liability claims in most countries (Reuters)
- Zostavax Lawsuit (ClassAction.com)
- Pfizer to pay £50m after deaths of Nigerian children in drug trial experiment (The Independent)
- MHRA urgently seeks software tool to process the expected high volume of Covid-19 vaccine Adverse Drug Reaction (Tenders Electronic Daily)
- Pfizer given protection from legal action by UK government (The Independent)
View all articles related to COVID-19 and vaccination.
Mexican health officials are investigating after two babies died and 29 children were hospitalized from suspected adverse reactions to shots from the country’s national vaccination program.
Six of the children hospitalized in the southern Mexican state of Chiapas remain in serious condition, the Mexican Social Security Institute said on Sunday.
The illnesses were reported after 52 children from the rural mining community of La Pimienta were given vaccines Friday for tuberculosis, rotavirus and Hepatitis B, the institute said. Later that night, 31 of the children “presented adverse reactions presumably associated with the application of these vaccines,” officials said. Two of the children later died.
https://edition.cnn.com/2015/05/10/health/mexico-vaccine-deaths/index.html
Dozens of British children who developed narcolepsy as a result of a swine flu vaccine could be compensated after the high court rejected a government appeal to withhold payments.
Six million people in Britain, and more across Europe, were given the Pandemrix vaccine made by GlaxoSmithKline during the 2009-10 swine flu pandemic, but the jab was withdrawn after doctors noticed a sharp rise in narcolepsy among those who received it.