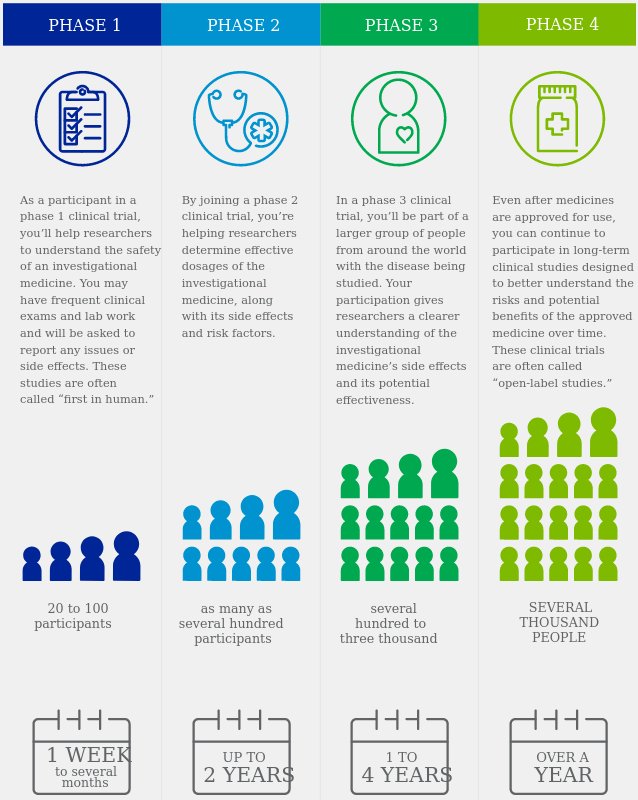
The process of learning about and developing an investigational medicine is divided into four phases. At first, very few people receive the medicine being studied. The number of people participating in clinical studies grows along with our understanding of the investigational medicine, and the research continues as long as the potential benefits outweigh the risks.
https://www.pfizer.com/science/clinical-trials/guide-to-clinical-trials/phases